LQTS
Auteur: Louise R.A. Olde Nordkamp
Supervisor: Arthur A.M. Wilde
The Long QT Syndrome (LQTS) refers to a condition in which there is an abnormally long QT interval on the ECG. This was first recognized by Dr. Jervell and Dr. Lange-Nielsen in 1957. They described 4 children with a long QT interval which was accompanied by hearing deficits, sudden cardiac death and an autosomal recessive inheritance. The LQTS may be divided into two distinct forms: congenital LQTS and acquired LQTS. These forms may however overlap when QT prolongation due to medication occurs in a patient with congenital LQTS.
Diagnosis
General
- The diagnosis is by measurement of the heart rate corrected QT interval on the ECG, which can be calculated with the QTc calculator.
- Sometimes the QT interval can be difficult to assess. Read the guidelines for measurement of difficult QT interval.
- A QTc of > 500ms in patients with Long QT Syndrome is associated with an increased risk of torsade de pointes and sudden death.[1]
- In patients suspected of LQTS (e.g. family members of known genotyped LQTS patients) a QTc > 430ms makes it likely that a LQTS gene defect is present.
- Because the QTc can change with age, it is best to take the ECG with the longest QTc interval (without additional QT-prologing factors) for risk stratification.
Physical examination
Patients can present with symptoms of arrhythmias:
- Fast or slow heart beat
- Weakness, lightheadedness, dizziness, syncope
- Chest pain
- Shortness of breath
- Paleness
- Sweating
Acquired LQTS
Acquired LQTS is most often caused by drugs that prolong the QT interval; combined with risk factors the risk of Torsade de Pointes is likely to increase.
Notorious QT prolonging drugs: |
|---|
|
Concomittant risk factors for medication induced torsade de pointes: |
|---|
|
Congenital LQTS
The prevalence of congenital LQTS is about 1:2000-2500. More than 10 different types of congenital LQTS have been described. However, only LQTS 1-3 are relatively common.
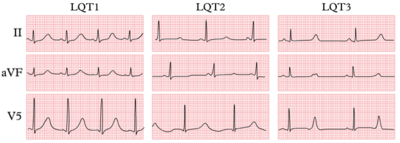
The three most common forms of LQTS can be recognized by the characteristic clinical features and ECG abnormalities
| LQTS type | LQTS 1 | LQTS 2 | LQTS 3 |
|---|---|---|---|
| Gene/current | KCNQ1/IKs | KCNH2/IKr | SCN5A/INa |
| B-blokker efficacy | ++++ | +++ | ++ |
| ECG | Early onset broad based T wave | Small late T wave | Late onset T wave with normal configuration |
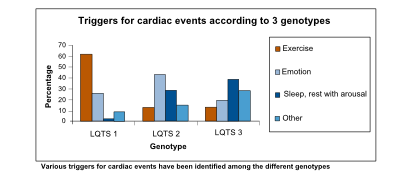
| Arrhythmogenic triggers | Exercise, especially swimming | Adrenergic triggers, especially nightly noise | Rest |
| Number of mutation carriers with events at age <15 | 40% | 20% | 10% |
| Number of mutation carriers with events at age <40 | 60% | 60% | 50% |
| Sudden cardiac death incidence Eponyme | 0,30% / year | 0,60% / year | 0,56% / year |
| Eponyme | If condition is homozygous: Jervell and Lange-Nielsen syndrome 1 |
Before the genes involved were known, some syndromes associated with a prolonged QT interval on the ECG had been described earlier:
- Anton Jervell and Fred Lange-Nielsen from Oslo described in 1957 an congenital syndrome that was associated with QT interval prolongation, deafness and sudden death: the now called Jervell-Lange-Nielsen syndrome.
- Romano-Ward syndrome is a long QT syndrome with normal auditory function and autosomal dominant inheritance.[2]
- Andersen-Tawil syndrome was described in 1994 by Tawil et al. and was associated with potassium-sensitive periodic paralysis, ventricular ectopy and dysmorphic features (short stature, low-set ears, hypoplastic mandible, clinodactyly and scoliosis). It later appeared to be associated with a mutation in the KCNJ2 gene (LQTS type 7).
- Timothy syndrome is a LQTS syndrome (with frequently alternating T-waves) with webbing of fingers and toes, congenital heart disease, immune deficiency, intermittent hypoglycaemia, cognitive abnormalities and autism. It appeared to be caused by a single mutation in the CACNA1C gene (LQTS type 8).
| Findings | Points | ||
| ECG | QTc: | >480 ms
460-479 450-459 (in males) >480 during X-ECG |
3
2 1 1 |
| Torsade de pointes
T-wave alternans Notched T-wave in 3 leads Low heart rate for age (<2nd percentile for age) |
2
1 1 0.5 | ||
| Syncope: | With stress
Without stress |
2
1 | |
| Clinical history | Congenital deafness
Family members with definite LQTS |
0.5
1 | |
| Family history | Unexplained sudden cardiac death before age 30 | 0.5 | |
| Total score ≤1 Low probability; 1.5-3 Intermediate probability; ≥3.5 High probability | |||
Diagnostic criteria by Schwartz et al. (2011)[3]
Clinical diagnosis
Diagnosis of LQTS is established by prolongation of the QTc interval in the absence of specific conditions known to lengthen it (for example QT-prolonging drugs) and/or molecular genetic testing of genes associated with LQTS.
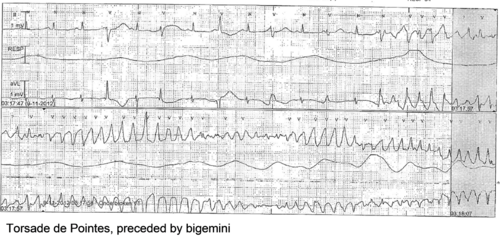
The prolonged QT interval can cause torsades de pointes, which is usually self-terminating, thus potentially causing a cardiac syncopal event. In LQTS type 1, cardiac symptoms are often precipitated by exercise; especially swimming is notorious for life-threatening cardiac events. In LQTS type 2, arrhythmogenic triggers are adrenergic; especially nightly noise (such as the morning alarm clock or nightly thunderlightening) is known to cause life-threatening cardiac events. On the other hand, in LQTS type 3, QT prolongation and possibly subsequent torsade de pointes is precipitated by bradycardia.
ECG tests
ECGs can be difficult because there is a considerable overlap between the QT interval of affected and unaffected individuals.
- The resting ECG is neither completely sensitive nor specific for the diagnosis of LQTS. The diagnostic criteria for the resting ECG are shown above in the list of diagnostic criteria by Schwartz et al. Besides a prolonged QTc, the T-wave can have different patterns among the different genotypes.
- Holter recordings appear to be of minimal clinical utility from a diagnostic and prognostic prospective in evaluating LQTS
- The exercise ECG (X-ECG) commonly shows failure of the QT to shorten normally, thereby prolonging the corrected QT interval, and many individuals develop characteristic T-wave abnormalities.
- A brisk-standing test ECG, where the QT-interval is measured after abrupt standing with subsequent heart rate acceleration. There appears to be a form of QT-stretching and QT-stunning [4] as demonstrated by Viskin et al. and Adler et al.
- Epinephrine infusion is a provocative test that might increase the sensitivity of the ECG findings. However, especially the negative predictive value is high.
- Adenosine infusion is a test provoking transient bradycardia followed by sinus tachycardia and therefore triggers QT changes that can distinguish patients with LQTS from healthy controls[5]
Genetic diagnosis
Today, 14 LQTS genes associated with LQTS have been identified. Most commonly, KCNQ1, KCNH2 and SCN5A, which are associated with LQTS type 1, type 2 and type 3 respectively, are found. Other, less frequently involved genes are displayed the table below.
There is an important genotype-phenotype relationship on severity of the disease.[3] In genotype–phenotype studies in the Rochester LQTS registry it was shown that in both LQTS type 1 and type 2, mutation locations and the degree of ion channel dysfunction caused by the mutations are important independent risk factors influencing the clinical course of this disorder.
| LQTS type | Gene | Protein (Ionchannel) | OMIM-link |
|---|---|---|---|
| Type 1 | KCNQ1 | KVLQT1 (IKs) | 607542 |
| Type 2 | KCNH2 | HERG (IKr) | 613688 |
| Type 3 | SCN5A | Sodium channel (INa) | 603830 |
| Type 4 | ANK2 | Ankyrin B (INa, K) | 600919 |
| Type 5 | KCNE1 | minK (IKs) | 176261 |
| Type 6 | KCNE2 | MiRP1 (IKr) | 613693 |
| Type 7 | KCNJ2 | Kir 2.1 (IK1) | 170390 |
| Type 8 | CACNA1C | Cav1.2 (ICa-L) | 601005 |
| Type 9 | CAV3 | Caveolin-3 | 601253 |
| Type 10 | SCN4B | Sodium channel (INa) | 608256 |
| Type 11 | AKAP9 | A-Kinase anchor 9 (IKs) | 604001 |
| Type 12 | SNTA1 | Syntrophin (INa) | 601017 |
| Type 13 | KCNJ5 | Kir3.4 (IK) | 600734 |
| Type 14 | CALM1 | Calmodulin 1 | 114180 |
Risk Stratification
Gene-specific differences of the natural history of LQTS have also been demonstrated and allow genotype-based risk stratification. Indeed, QT interval duration, gender and genotype (including mutation location and degree of ion channel dysfunction) are significantly associated with the outcome, with a QTc interval >500ms, and a LQT2 or LQT3 genotype determining the worst prognosis. Gender differently modulates the outcome according to the underlying genetic defect: the LQT3 males and LQT2 females are the highest risk subgroups. Risk stratification is best done by an expert cardio-genetics cardiologist.
Treatment
"Lifestyle modification":
- Probably no competitive sports in all LQTS patients
- Avoid QT-prolonging drugs in all LQTS patients
- No swimming or diving in LQT1 patients
- Avoid nightly or sudden noise in LQT2 patients (e.g. no alarm clock)
Medication/Other therapies:
- Beta-blockers are the cornerstone of therapy in LQTS. Beta-blockers even reduce the risk of sudden death in patients in whom a genetic defect has been found, but no QT prolongation is visible on the ECG and also in LQTS3 patients with bradycardia-associated cardiac events [6]
- ICD implantation in combination with beta-blockers in LQTS patients with previous cardiac arrest, cardiac syncope or ventricular tachycardia while on beta-blockers. ICDs should have pacing possibilities, because arrhythmic episodes are bradycardia-associated in LQTS type 3 and since post-shock pacing is relevant in all other LQTS types.
- Cardiac sympathetic denervation (LCSD) should be considered in the setting of beta-blocker breakthroughs, intolerance to pharmacotherapy and history of appropriate ICD therapies. Surgically, LCSD involves the resection of the lower two-third of the left stellate ganglion and the left-sided sympathetic chain at the level of T2, T3 and T4.
References
Error fetching PMID 12736279:
Error fetching PMID 19926013:
Error fetching PMID 17470695:
Error fetching PMID 20301308:
Error fetching PMID 22042885:
Error fetching PMID 22083145:
Error fetching PMID 20116193:
Error fetching PMID 22300664:
Error fetching PMID 15851169:
Error fetching PMID 16105845:
- Error fetching PMID 12736279:
- Error fetching PMID 20301308:
- Error fetching PMID 11136691:
- Error fetching PMID 22300664:
- Error fetching PMID 16105845:
- Error fetching PMID 15851169:
- Error fetching PMID 19926013:
- Error fetching PMID 17470695:
- Error fetching PMID 22042885:
- Error fetching PMID 22083145:
- Error fetching PMID 20116193: