Grown-up Congenital Heart Disease (GUCH): Difference between revisions
| (28 intermediate revisions by the same user not shown) | |||
| Line 66: | Line 66: | ||
:: Muscular defects (type 4) are located within the trabecular septum and accounts for 5 – 20% of all VSDs. It is bordered only by muscle, away from the cardiac valves. Muscular defects can be small or large in size and consist of a single or multiple defects. | :: Muscular defects (type 4) are located within the trabecular septum and accounts for 5 – 20% of all VSDs. It is bordered only by muscle, away from the cardiac valves. Muscular defects can be small or large in size and consist of a single or multiple defects. | ||
==== | ==== Pathophysiology ==== | ||
The severity of the shunt across the VSD is determined by its size and the ratio of pulmonary to systemic vascular resistance. In small or restrictive VSDs the diameter of the defect is ≤25% of the aortic annulus diameter. These small defects cause small left to right shunts with no left ventricular overload or pulmonary hypertension. | The severity of the shunt across the VSD is determined by its size and the ratio of pulmonary to systemic vascular resistance. In small or restrictive VSDs the diameter of the defect is ≤25% of the aortic annulus diameter. These small defects cause small left to right shunts with no left ventricular overload or pulmonary hypertension. | ||
| Line 74: | Line 74: | ||
In large defects, defined as those with diameters equal or greater than 75% of the aortic annulus, there is no restriction of blood flow across the septum, leading to equal pressures in both right and left ventricle. The large left to right shunt initially only leads to excessive volume overload in the pulmonary arteries, left atrium and left ventricle. The chronic pressure and volume overload combined with the increase flow leads to irreversible changes of the pulmonary vasculature, which results in an increase in pulmonary vascular resistance. This increase in resistance leads to a reversal of the shunt through the VSD causing right to left shunt with cyanosis (Eisenmenger syndrome). | In large defects, defined as those with diameters equal or greater than 75% of the aortic annulus, there is no restriction of blood flow across the septum, leading to equal pressures in both right and left ventricle. The large left to right shunt initially only leads to excessive volume overload in the pulmonary arteries, left atrium and left ventricle. The chronic pressure and volume overload combined with the increase flow leads to irreversible changes of the pulmonary vasculature, which results in an increase in pulmonary vascular resistance. This increase in resistance leads to a reversal of the shunt through the VSD causing right to left shunt with cyanosis (Eisenmenger syndrome). | ||
==== | ==== Evaluation ==== | ||
| Line 84: | Line 84: | ||
With echocardiography the localisation, size and hemodynamic influence of the VSD can be investigated. Dilatation of the left atrium and left ventricle might be present and the pressures in the pulmonary artery can be estimated by means of the tricuspid regurgitation. Invasive measurement by means of catheterization is only indicated when there is doubt about the shunt size and the pulmonary vascular resistance. | With echocardiography the localisation, size and hemodynamic influence of the VSD can be investigated. Dilatation of the left atrium and left ventricle might be present and the pressures in the pulmonary artery can be estimated by means of the tricuspid regurgitation. Invasive measurement by means of catheterization is only indicated when there is doubt about the shunt size and the pulmonary vascular resistance. | ||
==== | ==== Treatment ==== | ||
Treatment and prognosis of a VSD depends on the size en localisation of the defect, the pulmonary vascular resistance and possible concomitant defects. Spontaneous closure occurs mainly in small defects, of which 75 percent closes before age 10. In patients with a small defect no pulmonary hypertension develops, however there is an increased risk of endocarditis. | Treatment and prognosis of a VSD depends on the size en localisation of the defect, the pulmonary vascular resistance and possible concomitant defects. Spontaneous closure occurs mainly in small defects, of which 75 percent closes before age 10. In patients with a small defect no pulmonary hypertension develops, however there is an increased risk of endocarditis. | ||
| Line 93: | Line 93: | ||
Repair of VSD has been historically performed surgically. However, percutaneous VSD repair has been growing given the desire of young adults to avoid surgery. Surgical and percutaneous VSD closure should be performed by surgeons and cardiologists with appropriate training and expertise. | |||
Indications for closure of a VSD in an adult are included in the 2008 American College of Cardiology/American Heart Association (ACC/AHA) adult congenital heart disease guidelines as follows. Similar recommendations are included in the European Society of Cardiology and the Canadian Society of Cardiology guidelines. | |||
* Closure of a VSD is indicated when there is a Qp/Qs ≥2 and clinical evidence of LV volume overload. | * Closure of a VSD is indicated when there is a Qp/Qs ≥2 and clinical evidence of LV volume overload. | ||
* Closure of a VSD is indicated when the patient has a history of infective endocarditis. | * Closure of a VSD is indicated when the patient has a history of infective endocarditis. | ||
| Line 208: | Line 208: | ||
=== Case report === | === Case report === | ||
=== Introduction === | === Introduction === | ||
[[File:9. | [[File:Figure 9. Schematic drawing of the anatomy prenatal and postnatal.png|thumb|right|Figure 9. Schematic drawing of the anatomy prenatal (left) and postnatal (right) in coarctation of the aorta. In the normal situation (without coarctation) only 10 percent of the fetal cardiac output flows through the descending aorta. Therefore there are no hemodynamic consequences prenatal of coarctation of the aorta. In the postnatal situation, after closure of the ductus arteriosus, around 75% of cardiac output needs to pass the coarctation, leading to obstruction.]] | ||
Coarctation of the aorta is a narrowing of the thoracic aorta, typically located in the region of the obliterated ductus arteriosum. (Figure 9) The relation to the position of the left subclavian artery differs, in most patients the left subclavian artery is located anterior of the coarctation. Aortic coarctation is frequently associated with diffuse hypoplasia of the aortic arch and isthmus. | Coarctation of the aorta is a narrowing of the thoracic aorta, typically located in the region of the obliterated ductus arteriosum. (Figure 9) The relation to the position of the left subclavian artery differs, in most patients the left subclavian artery is located anterior of the coarctation. Aortic coarctation is frequently associated with diffuse hypoplasia of the aortic arch and isthmus. | ||
| Line 235: | Line 235: | ||
=== Treatment and outcome === | === Treatment and outcome === | ||
[[File:10. | [[File:Figure 10. Schematic drawing showing surgical procedures for repair of coarctation of the aorta.png|thumb|left|Figure 10. Schematic drawing showing surgical procedures for repair of coarctation of the aorta. Left: resection with end-to-end anastomosis. Middle: dilating technique using a patch; this technique is used in coarctations involving a long segment of the aorta. Right: the subclavian flap aortoplasty, using the left subclavian artery.]] | ||
[[File:11. | [[File:Figure 11. Schematic drawing showing surgical procedures for repair of a coarctation of the aorta.png|thumb|right|Figure 11. Schematic drawing showing surgical procedures for repair of a coarctation of the aorta. Left: an interposition graft. Middle: the extended aortic arch repair. Right: the extra-anatomical bypass.]] | ||
<!-- | <!-- | ||
{{multiple image | {{multiple image | ||
| Line 260: | Line 260: | ||
=== Introduction === | === Introduction === | ||
[[File:12. TGA.jpg|thumb|left|Figure 12: Schematic drawing showing transposition of the great arteries. The pulmonary artery is located above the left ventricle (LV) and the aorta is located above the right ventricle (RV).]] | [[File:12. TGA.jpg|thumb|left|Figure 12: Schematic drawing showing transposition of the great arteries. The pulmonary artery is located above the left ventricle (LV) and the aorta is located above the right ventricle (RV).]] | ||
[[File:13. | [[File:Figure 13. Schematic drawing of the circulation in transposition of the great arteries.png|thumb|right|Figure 13. Schematic drawing of the circulation in transposition of the great arteries. Left: normal position of the great arteries with the pulmonary and systemic circulation serially connected. Right: transposition of the great arteries with a parallel circulation.]] | ||
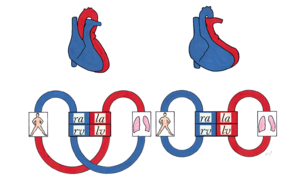
Transposition of the great arteries (TGA) accounts for 5-8% of all congenital heart defects and occurs 2-3 times more frequently in males. TGA is best defined as a normal atrioventricular connection with an abnormal ventricular–arterial connection; the morphological left atrium is connected through the left ventricle with the pulmonary artery and the morphological right atrium through the right ventricle with the aorta. (Figure 12)The aorta is often located on the right side and in front of the pulmonary artery (D-TGA). In 70 percent there is an isolated form of TGA, in 30 percent the TGA is accompanied by other heart defects, like VSD or obstruction of the left ventricle outflow tract. | Transposition of the great arteries (TGA) accounts for 5-8% of all congenital heart defects and occurs 2-3 times more frequently in males. TGA is best defined as a normal atrioventricular connection with an abnormal ventricular–arterial connection; the morphological left atrium is connected through the left ventricle with the pulmonary artery and the morphological right atrium through the right ventricle with the aorta. (Figure 12)The aorta is often located on the right side and in front of the pulmonary artery (D-TGA). In 70 percent there is an isolated form of TGA, in 30 percent the TGA is accompanied by other heart defects, like VSD or obstruction of the left ventricle outflow tract. | ||
=== Pathophysiology === | === Pathophysiology === | ||
The circulation in TGA patients is not serial but parallel ( | The circulation in TGA patients is not serial but parallel (Figure 13); the venous blood is returned to the systemic circulation through the right atrium and ventricle, while the arterial oxygenated blood is directed back into the pulmonary artery through the left atrium and ventricle. Due to this abnormal circulation there is severe cyanosis directly after birth, therefore it is critical for the ductus arteriosus and foramen ovale to remain open. Without treatment there is a mortality of 30% within one week, 50% within one month and 90% within one year. When an associated VSD is present the chances of survival are higher due to more shunting thus more oxygenated blood in the systemic circulation. These patients are able to reach early adulthood without corrective surgery or intervention. However the pulmonary hypertension that develops in this situation will eventually lead to severe problems. | ||
=== Treatment === | === Treatment === | ||
| Line 282: | Line 282: | ||
=== Congenitally corrected transposition of the great arteries === | === Congenitally corrected transposition of the great arteries === | ||
=== Introduction === | === Introduction === | ||
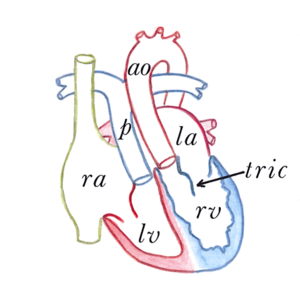
[[File:14. | [[File:Figure 14. Congenitally corrected transposition of the great arteries.png|thumb|right|Figure 14. Congenitally corrected transposition of the great arteries. RA, right atrium. LA, left atrium. RV, right ventricle. LV, left ventricle. p, pulmonary artery. ao, aorta. tric, tricuspid valve.]] | ||
The congenitally corrected transposition of the great arteries (ccTGA) is characterized by a normal anatomical position of both atria, with an abnormal connection between the atria and the ventricles. The right atrium is connected with the left ventricle and the left atrium is connected with the right ventricle. (Figure 14) Furthermore the aorta arises from the right ventricle and the pulmonary artery from the left ventricle. There are, in conclusion, abnormal atrioventricular connections and abnormal ventricular-arterial connections present in ccTGA. | The congenitally corrected transposition of the great arteries (ccTGA) is characterized by a normal anatomical position of both atria, with an abnormal connection between the atria and the ventricles. The right atrium is connected with the left ventricle and the left atrium is connected with the right ventricle. (Figure 14) Furthermore the aorta arises from the right ventricle and the pulmonary artery from the left ventricle. There are, in conclusion, abnormal atrioventricular connections and abnormal ventricular-arterial connections present in ccTGA. | ||
CcTGA is a very rare defect, accounting for about 1% of all congenital heart disease. | CcTGA is a very rare defect, accounting for about 1% of all congenital heart disease. | ||
| Line 325: | Line 325: | ||
=== Pathophysiology === | === Pathophysiology === | ||
[[File:15. HLHS.svg|thumb|left|Figure 15. Schematic drawing representing the hypoplastic left heart syndrome.]] | |||
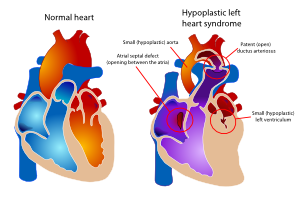
The hypoplastic left heart syndrome (HLHS) is the most common type of univentricular heart. (Figure 15) Not only the left ventricle, but often the aortic valve, ascending aorta and aortic arch are hypoplastic as well. This will redirect blood from the left atrium into the right atrium, where is will be mixed with venous blood and pumped into the right ventricle and pulmonary artery. The whole systemic circulation depends on the shunt from pulmonary artery through the ductus arteriosus into the aorta. When the ductus starts closing the consequences are dramatic, with severe cyanosis and acidosis. | |||
The hypoplastic left heart syndrome (HLHS) is the most common type of univentricular heart. ( | [[File:24. univentricular heart.PNG|thumb|right|Figure 24. Echocardiographic image of a male patient with a univentricular heart.]] | ||
When a hypoplastic right ventricle is present with associated atresia of the pulmonary artery, the pulmonary circulation after birth will solely depend on the left-to-right shunt through the ductus arteriosus.When the ductus starts closing, progressive cyanosis is the main presenting symptom. | When a hypoplastic right ventricle is present with associated atresia of the pulmonary artery, the pulmonary circulation after birth will solely depend on the left-to-right shunt through the ductus arteriosus.When the ductus starts closing, progressive cyanosis is the main presenting symptom. | ||
| Line 334: | Line 335: | ||
All patients with one functioning ventricle have complete mixing of saturated and desaturated blood leading to chronic hypoxemia ( | All patients with one functioning ventricle have complete mixing of saturated and desaturated blood leading to chronic hypoxemia (Figure 24). Furthermore there is a chronic volume overload to the ventricle, serving as both pulmonary and systemic ventricle, leading to an early development of heart failure. | ||
Due to the obligatory intracardiac shunting the pulmonary ‘filter’ is bypassed, which will increase the chance of cardiovascular accidents and brain abscesses. | Due to the obligatory intracardiac shunting the pulmonary ‘filter’ is bypassed, which will increase the chance of cardiovascular accidents and brain abscesses. | ||
=== Treatment === | === Treatment === | ||
[[File:16. Fontan.svg|thumb|left|Figure 16. Schematic drawing showing the Fontan procedure.]] | |||
In case of a ductus-dependent defect initial treatment immediately after birth consists of prevention of ductus closure. At first this can be achieved pharmacologically with prostaglandin, however due to the many side effects this is no long-term solution. | In case of a ductus-dependent defect initial treatment immediately after birth consists of prevention of ductus closure. At first this can be achieved pharmacologically with prostaglandin, however due to the many side effects this is no long-term solution. | ||
When there is a dependent pulmonary circulation an aortopulmonary shunt will be constructed during the first weeks of life to ensure accurate blood flow to the lungs after discontinuation of the prostaglandin. | When there is a dependent pulmonary circulation an aortopulmonary shunt will be constructed during the first weeks of life to ensure accurate blood flow to the lungs after discontinuation of the prostaglandin. | ||
If there is a dependent systemic circulation the surgical treatment usually consists of three different steps. Since the anatomy is by no means normalized, one can not speak of a surgical correction, it is referred to as a definitive palliation. At first a Norwood or Sano procedure is performed in neonates where a neo-aorta is constructed by dividing the pulmonary artery. Second stage is the construction of a cavopulmonary shunt, also known as bidirectional Glenn shunt, which is performed at 4 -6 months of age. The third and final stage is known as Fontan procedure and performed at 18 – 30 months of age, where a total cavopulmonary connection is created. ( | If there is a dependent systemic circulation the surgical treatment usually consists of three different steps. Since the anatomy is by no means normalized, one can not speak of a surgical correction, it is referred to as a definitive palliation. At first a Norwood or Sano procedure is performed in neonates where a neo-aorta is constructed by dividing the pulmonary artery. Second stage is the construction of a cavopulmonary shunt, also known as bidirectional Glenn shunt, which is performed at 4 -6 months of age. The third and final stage is known as Fontan procedure and performed at 18 – 30 months of age, where a total cavopulmonary connection is created. (Figure 16) All surgical procedures are described in more detail separately. | ||
=== Outcome === | === Outcome === | ||
| Line 367: | Line 368: | ||
=== Case report === | === Case report === | ||
=== Introduction === | === Introduction === | ||
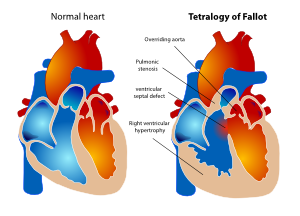
[[File:17. TOF.svg|thumb|right|Figure 17. Schematic drawing representing the four features of tetralogy of Fallot.]] | |||
In 1888 Etienne Louis Arthur Fallot described the ‘maladie bleue’ as a combination of: | In 1888 Etienne Louis Arthur Fallot described the ‘maladie bleue’ as a combination of: | ||
| Line 375: | Line 376: | ||
* Hypertrophic right ventricle | * Hypertrophic right ventricle | ||
This constellation of findings has since become known as tetralogy of Fallot (TOF). ( | This constellation of findings has since become known as tetralogy of Fallot (TOF). (Figure 17) | ||
The prevalence of TOF is about 3.9 per 10.000 live births. This defect accounts for about 7 to 10 percent of cases of congenital heart disease and is one of the most common congenital heart lesions requiring intervention in the first year of life. | The prevalence of TOF is about 3.9 per 10.000 live births. This defect accounts for about 7 to 10 percent of cases of congenital heart disease and is one of the most common congenital heart lesions requiring intervention in the first year of life. | ||
| Line 396: | Line 397: | ||
Patients with TOF can undergo either palliative (shunts) or corrective (intracardiac repair) surgery. Although most children with TOF undergo intracardiac repair as their initial intervention, the principle of shunts remains an important palliative procedure for infants who may not be acceptable candidates for intracardiac repair due to prematurity, hypoplastic pulmonary arteries, or coronary artery anatomy. | Patients with TOF can undergo either palliative (shunts) or corrective (intracardiac repair) surgery. Although most children with TOF undergo intracardiac repair as their initial intervention, the principle of shunts remains an important palliative procedure for infants who may not be acceptable candidates for intracardiac repair due to prematurity, hypoplastic pulmonary arteries, or coronary artery anatomy. | ||
Shunts are constructed to increase the blood flow to the lungs, to improve the development of the pulmonary arteries. Many patients who underwent intracardiac repair initially had a palliative shunt. Blalock and Taussig first reported successful surgical palliation of TOF in 1945. The procedure, which has since come to bear their names, used a subclavian artery to create an aorta-to-pulmonary artery connection. The technique has been modified and is now usually performed using a Gortex tube to create the connection. | |||
A different type of shunt is the aortopulmonary anastomis, where a direct connection between the descending aorta and left pulmonary artery (Potts) or between the ascending aorta and the right pulmonary artery (Waterston) is constructed. | |||
Intracardiac repair of TOF was reported by Lillehi in 1954. It consists of patch closure of the ventricular septal defect and enlargement of the RVOT. The latter is accomplished by relieving pulmonary stenosis, resecting infundibular and subinfundibular muscle bundles and if necessary by a transannular patch, creating unobstructed flow from the RV into the pulmonary arteries. | Intracardiac repair of TOF was reported by Lillehi in 1954. It consists of patch closure of the ventricular septal defect and enlargement of the RVOT. The latter is accomplished by relieving pulmonary stenosis, resecting infundibular and subinfundibular muscle bundles and if necessary by a transannular patch, creating unobstructed flow from the RV into the pulmonary arteries. | ||
=== Outcome === | === Outcome === | ||
| Line 417: | Line 418: | ||
=== Case report === | === Case report === | ||
=== Introduction === | === Introduction === | ||
[[File:18. MFS.jpg|thumb|left|Figure 18. Echocardiographic image of aortic root dilatation in Marfan syndrome.]] | |||
[[File:19. MFS2.jpg|thumb|right|Figure 19. Magnetic resonance imaging of the aorta, showing aortic root dilatation in Marfan syndrome.]] | |||
Marfan syndrome (MFS) is an autosomal dominant condition with a reported incidence of 1 in 3000 to 5000 individuals and is one of the most common inherited disorders of connective tissue. While most MFS patients have an affected parent, around 15 – 30 percent have a de novo mutation. MFS is associated with a broad range of clinical symptoms and associated disorders, ranging from classic ocular, cardiovascular, and musculoskeletal abnormalities to manifestations including involvement of the lung, skin, and central nervous system. | Marfan syndrome (MFS) is an autosomal dominant condition with a reported incidence of 1 in 3000 to 5000 individuals and is one of the most common inherited disorders of connective tissue. While most MFS patients have an affected parent, around 15 – 30 percent have a de novo mutation. MFS is associated with a broad range of clinical symptoms and associated disorders, ranging from classic ocular, cardiovascular, and musculoskeletal abnormalities to manifestations including involvement of the lung, skin, and central nervous system. | ||
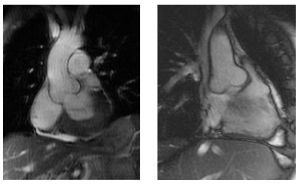
Progressive dilatation of the ascending aorta is one of the key features, which causes a high risk of sudden death due to aortic dissection or rupture in young Marfan patients. ( | Progressive dilatation of the ascending aorta is one of the key features, which causes a high risk of sudden death due to aortic dissection or rupture in young Marfan patients. (Figure 18 & 19) | ||
The underlying genetic defect is localised in the fibrillin gene on chromosome 15 (FBN1) in which recently around 600 different mutations are found. However in about 10% of MFS patients there is no mutation identified in the FBN1 gene, furthermore FBN1 mutations also occur across a wide range of milder phenotypes that overlap the classic Marfan phenotype. Therefore it is not possible to diagnose MFS solely with genetic information. | The underlying genetic defect is localised in the fibrillin gene on chromosome 15 (FBN1) in which recently around 600 different mutations are found. However in about 10% of MFS patients there is no mutation identified in the FBN1 gene, furthermore FBN1 mutations also occur across a wide range of milder phenotypes that overlap the classic Marfan phenotype. Therefore it is not possible to diagnose MFS solely with genetic information. | ||
| Line 470: | Line 472: | ||
Beta blockers decrease myocardial contractility and may also improve the elastic properties of the aorta, particularly in patients with an aortic root diameter <40 mm thereby decreasing the risk of aortic dissection and delaying the aortic dilatation. Prophylactic treatment with beta blockers is considered the standard of care in adults with MFS. Furthermore patients with MFS are advised to avoid any contact sports, exercise at maximal capacity, and isometric activities. | Beta blockers decrease myocardial contractility and may also improve the elastic properties of the aorta, particularly in patients with an aortic root diameter <40 mm thereby decreasing the risk of aortic dissection and delaying the aortic dilatation. Prophylactic treatment with beta blockers is considered the standard of care in adults with MFS. Furthermore patients with MFS are advised to avoid any contact sports, exercise at maximal capacity, and isometric activities. | ||
The exact aortic root diameter at which elective surgery should be performed is uncertain. The current guidelines recommend elective operation for patients with MFS at an external diameter of ≥50 mm to avoid acute dissection or rupture. Indications for repair at an external diameter less than 50 mm include rapid growth (>2 mm/y), family history of aortic dissection at a diameter less than 50 mm, desire of pregnancy or presence of progressive aortic or mitral valve regurgitation. However one must take into account that a predicted aortic root diameter varies with body size and age and may be smaller in women. Smaller patients have dissection at a smaller aortic root size and 15 percent of patients with MFS have dissection at a diameter less than 50 mm. | |||
The classic aortic root surgery is the Bentall procedure in which the ascending aorta is replaced, together with the aortic valve, by a graft with prosthetic valve. In this procedure the coronary arteries need to be reimplanted in the aortic graft. In patients with anatomically normal valves, in whom the insufficiency is due to the dilated annulus or dissection, valve-sparing operations with root replacement by a Dacron prosthesis and with reimplantation of the coronary arteries into the prosthesis (David’s procedure) or remodelling of the aortic root (Yacoub’s procedure) have now become the preferred surgical procedures. Aortic regurgitation is, however, a common complication, requiring reoperation in 20% of patients after 10 years. | |||
=== Outcome === | === Outcome === | ||
| Line 481: | Line 483: | ||
=== Case report === | === Case report === | ||
=== Introduction === | === Introduction === | ||
Ebsteins anomaly, named after Wilhelm Ebstein (1836 – 1912) ( | [[File:20. Wilhelm Ebstein.jpg|thumb|left|Figure 20. Wilhelm Ebstein (1836 – 1912).]] | ||
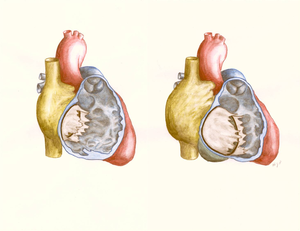
[[File:Figure 21. Schematic drawing showing Ebstein’s anomaly of the tricuspid valve.png|thumb|right|Figure 21. Schematic drawing showing Ebstein’s anomaly of the tricuspid valve. Left: normal heart with openend right ventricle. Right: Ebstein’s anomaly with displacement of the septal and posterior tricuspid leaflet, leading to atrialisation of a significant part of the right ventricle.]] | |||
Ebsteins anomaly, named after Wilhelm Ebstein (1836 – 1912) (Figure 20) is a congenital heart defect of the morphological tricuspid valve. The prevalence of Ebstein's anomaly is about 1 in 50.000 – 200.000 with a similar incidence in both males and females. | |||
As its name clearly indicates, the tricuspid valve consists of three leaflets; anterosuperior, septal and inferior. The Ebstein’s anomaly consists of a variety of anatomical and functional abnormalities of the tricuspid valve. Typical features are: | As its name clearly indicates, the tricuspid valve consists of three leaflets; anterosuperior, septal and inferior. The Ebstein’s anomaly consists of a variety of anatomical and functional abnormalities of the tricuspid valve. Typical features are: | ||
| Line 526: | Line 530: | ||
PH is classified into five groups: | PH is classified into five groups: | ||
# Pulmonary arterial hypertension (PAH). This group consists of idiopathic PAH and PAH due to connective tissue diseases, HIV infection, portal hypertension, congenital heart disease, schistosomiasis, chronic hemolytic anemia, persistent pulmonary hypertension of the newborn, pulmonary veno-occlusive disease, drug- and toxin-induced PH and pulmonary capillary hemangiomatosis. | # Pulmonary arterial hypertension (PAH). This group consists of idiopathic PAH and PAH due to connective tissue diseases, HIV infection, portal hypertension, congenital heart disease, schistosomiasis, chronic hemolytic anemia, persistent pulmonary hypertension of the newborn, pulmonary veno-occlusive disease, drug- and toxin-induced PH and pulmonary capillary hemangiomatosis. | ||
# Pulmonary hypertension owing to left heart disease. PH due to systolic dysfunction, diastolic dysfunction, or valvular heart disease is included in this group. | # Pulmonary hypertension owing to left heart disease. PH due to systolic dysfunction, diastolic dysfunction, or valvular heart disease is included in this group. | ||
# Pulmonary hypertension owing to lung diseases or hypoxemia. This group includes PH due to chronic obstructive pulmonary disease, interstitial lung disease, other pulmonary diseases with a mixed restrictive and obstructive pattern, sleep-disordered breathing, alveolar hypoventilation disorders, and other causes of hypoxemia [4]. | # Pulmonary hypertension owing to lung diseases or hypoxemia. This group includes PH due to chronic obstructive pulmonary disease, interstitial lung disease, other pulmonary diseases with a mixed restrictive and obstructive pattern, sleep-disordered breathing, alveolar hypoventilation disorders, and other causes of hypoxemia [4]. | ||
# Chronic thromboembolic pulmonary hypertension. This group includes patients with PH due to thromboembolic occlusion of the proximal or distal pulmonary vasculature. | # Chronic thromboembolic pulmonary hypertension. This group includes patients with PH due to thromboembolic occlusion of the proximal or distal pulmonary vasculature. | ||
# Pulmonary hypertension with unclear multifactorial mechanisms. These patients have PH caused by hematologic disorders (eg, myeloproliferative disorders), systemic disorders (eg, sarcoidosis), metabolic disorders (eg, glycogen storage disease), or miscellaneous causes | # Pulmonary hypertension with unclear multifactorial mechanisms. These patients have PH caused by hematologic disorders (eg, myeloproliferative disorders), systemic disorders (eg, sarcoidosis), metabolic disorders (eg, glycogen storage disease), or miscellaneous causes | ||
| Line 543: | Line 543: | ||
=== Pathophysiology === | === Pathophysiology === | ||
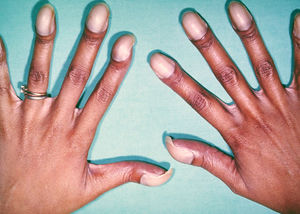
[[File:22. Eisenmenger.jpg|thumb|right|Figure 22. Photo showing typical features of chronic hypoxemia in Eisenmenger syndrome, with typical digital clubbing with cyanotic nail beds.]] | |||
The pathogenesis of PH is complex and just beginning to be elucidated. In patients with congenital heart disease, left-to-right intracardiac shunting increases flow through the pulmonary vasculature, this causes shear forces that disrupt the vascular endothelium and activate cellular mechanisms critical to the pathogenesis and progression of PAH. | The pathogenesis of PH is complex and just beginning to be elucidated. In patients with congenital heart disease, left-to-right intracardiac shunting increases flow through the pulmonary vasculature, this causes shear forces that disrupt the vascular endothelium and activate cellular mechanisms critical to the pathogenesis and progression of PAH. | ||
| Line 580: | Line 580: | ||
Therapy improves exercise capacity and functional class, however the impact on mortality has been less well established. | Therapy improves exercise capacity and functional class, however the impact on mortality has been less well established. | ||
== References == | |||
<biblio> | |||
#Basow2012a Basow, D. S. (2012a). Classification and clinical features of isolated atrial septal defects in children. UpToDate. Waltham, MA.: UpToDate. | |||
#Basow2012b Basow, D. S. (2012b). Management of atrial septal defects in adults. UpToDate. Waltham, MA.: UpToDate. | |||
#Basow2012c Basow, D. S. (2012c). Management and outcome of isolated atrial septal defects in children. UpToDate. Waltham, MA.: UpToDate. | |||
#Basow2012d Basow, D. S. (2012d). Pathophysiology and clinical features of atrial septal defects in adults. UpToDate. Waltham, MA.: UpToDate. | |||
#Basow2012e Basow, D. S. (2012e). Identification and assessment of atrial septal defects in adults. UpToDate. Waltham, MA.: UpToDate. | |||
#Basow2012f Basow, D. S. (2012f). Devices for percutaneous closure of a secundum atrial septal defect. UpToDate. Waltham, MA.: UpToDate. | |||
#Berger Berger, F., Vogel, M., Alexi-Meskishvili, V., & Lange, P. E. (1999). Comparison of results and complications of surgical and Amplatzer device closure of atrial septal defects. The Journal of Thoracic and Cardiovascular Surgery, 118(4), 674-678; discussion 678-680 | |||
#Engelfriet Engelfriet, P., Boersma, E., Oechslin, E., Tijssen, J., Gatzoulis, M. A., Thilén, U., Kaemmerer, H., e.a. (2005). The spectrum of adult congenital heart disease in Europe: morbidity and mortality in a 5 year follow-up period. The Euro Heart Survey on adult congenital heart disease. European Heart Journal, 26(21), 2325-2333. doi:10.1093/eurheartj/ehi396 | |||
#Mulder Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006a). Atrial Septal Defect. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum. | |||
#Roos-Hesselink Roos-Hesselink, J. W., Meijboom, F. J., Spitaels, S. E. C., van Domburg, R., van Rijen, E. H. M., Utens, E. M. W. J., Bogers, A. J. J. C., e.a. (2003). Excellent survival and low incidence of arrhythmias, stroke and heart failure long-term after surgical ASD closure at young age. A prospective follow-up study of 21-33 years. European Heart Journal, 24(2), 190-197. | |||
#Basow2012h Basow, D. S. (2012h). Pathophysiology and clinical features of isolated ventricular septal defects in infants and children. UpToDate. Waltham, MA.: UpToDate. | |||
#Basow2012i Basow, D. S. (2012i). Management of isolated ventricular septal defects in infants and children. UpToDate. Waltham, MA.: UpToDate. | |||
#Baumgartner Baumgartner, H., Bonhoeffer, P., De Groot, N. M. S., de Haan, F., Deanfield, J. E., Galie, N., Gatzoulis, M. A., e.a. (2010). ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). European Heart Journal, 31(23), 2915-2957. | |||
#Mulder2 Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006b). Ventricular Septal Defect. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum. | |||
#Verheugt Verheugt, C. L., Uiterwaal, C. S. P. M., Grobbee, D. E., & Mulder, B. J. M. (2008). Long-term prognosis of congenital heart defects: a systematic review. International Journal of Cardiology, 131(1), 25-32. | |||
#Mulder3 Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Patent Ductus Arteriosus. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum | |||
#Rudolph Rudolph, A. M. (1970). The changes in the circulation after birth. Their importance in congenital heart disease. Circulation, 41(2), 343-359. | |||
#Ijland Ijland, M. M., & Tanke, R. B. (2009). Aortic coarctation. Circulation, 120(13), 1294-1295. | |||
#Luijendijk1 Luijendijk, P, Boekholdt, S. M., Blom, N. A., Groenink, M., Backx, A. P., Bouma, B. J., Mulder, B. J. M., e.a. (2011). Percutaneous treatment of native aortic coarctation in adults. Netherlands Heart Journal: Monthly Journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation, 19(10), 436-439. | |||
#Luijendijk2 Luijendijk, Paul, Bouma, B. J., Vriend, J. W. J., Vliegen, H. W., Groenink, M., & Mulder, B. J. M. (2011). Usefulness of exercise-induced hypertension as predictor of chronic hypertension in adults after operative therapy for aortic isthmic coarctation in childhood. The American Journal of Cardiology, 108(3), 435-439. | |||
#Mulder4 Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Coarctation of the aorta. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum. | |||
#Vriend1 Vriend, J. W. J., & Mulder, B. J. M. (2005). Late complications in patients after repair of aortic coarctation: implications for management. International Journal of Cardiology, 101(3), 399-406. | |||
#Vriend2 Vriend, J. W. J., Oosterhof, T., & Mulder, B. (2005). Noninvasive imaging for the postoperative assessment of aortic coarctation patients. Chest, 127(6), 2295. | |||
#Drenthen Drenthen, W., Pieper, P. G., Ploeg, M., Voors, A. A., Roos-Hesselink, J. W., Mulder, B. J. M., Vliegen, H. W., e.a. (2005). Risk of complications during pregnancy after Senning or Mustard (atrial) repair of complete transposition of the great arteries. European Heart Journal, 26(23), 2588-2595. doi:10.1093/eurheartj/ehi472 | |||
#Mulder5 Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Transposition of the great arteries. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum. | |||
#vanderZedde van der Zedde, J., Oosterhof, T., Tulevski, I. I., Vliegen, H. W., & Mulder, B. J. M. (2005). Comparison of segmental and global systemic ventricular function at rest and during dobutamine stress between patients with transposition and congenitally corrected transposition. Cardiology in the Young, 15(2), 148-153. doi:10.1017/S1047951105000326 | |||
#Mulder6 Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Congenitally corrected transposition of the great arteries. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum. | |||
#Winlaw Winlaw, D. S., McGuirk, S. P., Balmer, C., Langley, S. M., Griselli, M., Stümper, O., De Giovanni, J. V., e.a. (2005). Intention-to-treat analysis of pulmonary artery banding in conditions with a morphological right ventricle in the systemic circulation with a view to anatomic biventricular repair. Circulation, 111(4), 405-411. | |||
#Winter1 Winter, M. M., Bouma, B. J., van Dijk, A. P. J., Groenink, M., Nieuwkerk, P. T., van der Plas, M. N., Sieswerda, G. T., e.a. (2008). Relation of physical activity, cardiac function, exercise capacity, and quality of life in patients with a systemic right ventricle. The American Journal of Cardiology, 102(9), 1258-1262. | |||
#Winter2 Winter, M. M., van der Bom, T., de Vries, L. C. S., Balducci, A., Bouma, B. J., Pieper, P. G., van Dijk, A. P. J., e.a. (2011). Exercise training improves exercise capacity in adult patients with a systemic right ventricle: a randomized clinical trial. European Heart Journal. | |||
#Winter3 Winter, M. M., van der Plas, M. N., Bouma, B. J., Groenink, M., Bresser, P., & Mulder, B. J. M. (2010). Mechanisms for cardiac output augmentation in patients with a systemic right ventricle. International Journal of Cardiology, 143(2), 141-146. | |||
#Basow2012 Basow, D. S. (2012). Hypoplastic left heart syndrome. UpToDate. Waltham, MA.: UpToDate. | |||
#Fontan Basow, D. S. (2012). Hypoplastic left heart syndrome. UpToDate. Waltham, MA.: UpToDate. | |||
#Mulder7 Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Univentricular heart and the Fontan circulation. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum. | |||
#Schuuring Schuuring, M. J., Vis, J. C., Bouma, B. J., van Dijk, A. P. J., van Melle, J. P., Pieper, P. G., Vliegen, H. W., e.a. (2011). Rationale and design of a trial on the role of bosentan in Fontan patients: Improvement of exercise capacity? Contemporary Clinical Trials, 32(4), 586-591. | |||
#vandenBosch van den Bosch, A. E., Roos-Hesselink, J. W., Van Domburg, R., Bogers, A. J. J. C., Simoons, M. L., & Meijboom, F. J. (2004). Long-term outcome and quality of life in adult patients after the Fontan operation. The American Journal of Cardiology, 93(9), 1141-1145. | |||
#Balci Balci, A., Drenthen, W., Mulder, B. J. M., Roos-Hesselink, J. W., Voors, A. A., Vliegen, H. W., Moons, P., e.a. (2011). Pregnancy in women with corrected tetralogy of Fallot: occurrence and predictors of adverse events. American Heart Journal, 161(2), 307-313. | |||
#Lillehei Lillehei, C. W., Cohen, M., Warden, H. E., Read, R. C., Aust, J. B., Dewall, R. A., & Varco, R. L. (1955). Direct vision intracardiac surgical correction of the tetralogy of Fallot, pentalogy of Fallot, and pulmonary atresia defects; report of first ten cases. Annals of Surgery, 142(3), 418-442. | |||
#Mulder8 Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Tetralogy of Fallot. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum. | |||
#Mulder9 Mulder, Barbara J M, & van der Wall, E. E. (2009). Tetralogy of Fallot: in good shape? The International Journal of Cardiovascular Imaging, 25(3), 271-275. | |||
#Oosterhof Oosterhof, T., Mulder, B. J. M., Vliegen, H. W., & de Roos, A. (2006). Cardiovascular magnetic resonance in the follow-up of patients with corrected tetralogy of Fallot: a review. American Heart Journal, 151(2), 265-272. | |||
#Vliegen Vliegen, H. W., van Straten, A., de Roos, A., Roest, A. A. W., Schoof, P. H., Zwinderman, A. H., Ottenkamp, J., e.a. (2002). Magnetic resonance imaging to assess the hemodynamic effects of pulmonary valve replacement in adults late after repair of tetralogy of fallot. Circulation, 106(13), 1703-1707. | |||
#Windhausen Windhausen, F., Boekholdt, S. M., Bouma, B. J., Groenink, M., Backx, A. P. C. M., de Winter, R. J., Mulder, B. J. M., e.a. (2011). Per-operative stent placement in the right pulmonary artery; a hybrid technique for the management of pulmonary artery branch stenosis at the time of pulmonary valve replacement in adult Fallot patients. Netherlands Heart Journal: Monthly Journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation, 19(10), 432-435. | |||
#deWitte de Witte, Piet, Aalberts, J. J. J., Radonic, T., Timmermans, J., Scholte, A. J., Zwinderman, A. H., Mulder, B. J. M., e.a. (2011). Intrinsic biventricular dysfunction in Marfan syndrome. Heart (British Cardiac Society), 97(24), 2063-2068. | |||
#Engelfriet2 Engelfriet, P., & Mulder, B. (2007). Is there benefit of beta-blocking agents in the treatment of patients with the Marfan syndrome? International Journal of Cardiology, 114(3), 300-302. | |||
#Mulder9 Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Marfan’s syndrome. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum. | |||
#Radonic Radonic, T, de Witte, P., Groenink, M., de Bruin-Bon, R., Timmermans, J., Scholte, A., van den Berg, M., e.a. (2011). Critical appraisal of the revised Ghent criteria for diagnosis of Marfan syndrome. Clinical Genetics. | |||
#Basow20122 Basow, D. S. (2012). Ebstein’s anomaly of the tricuspid valve. UpToDate. Waltham, MA.: UpToDate. | |||
#Carpentier Carpentier, A., Chauvaud, S., Macé, L., Relland, J., Mihaileanu, S., Marino, J. P., Abry, B., e.a. (1988). A new reconstructive operation for Ebstein’s anomaly of the tricuspid valve. The Journal of Thoracic and Cardiovascular Surgery, 96(1), 92-101. | |||
#Celermajer Celermajer, D. S., Bull, C., Till, J. A., Cullen, S., Vassillikos, V. P., Sullivan, I. D., Allan, L., e.a. (1994). Ebstein’s anomaly: presentation and outcome from fetus to adult. Journal of the American College of Cardiology, 23(1), 170-176. | |||
#Ebstein Ebstein W. (1866) Ueber einen sehr seltenen Fall von Insufficienz der Valvula tricuspidalis, bedingt durch eine angeborene hochgradige Missbildung. Arch Anat physiol;33:238. | |||
#Mulder10 Mulder, B. J. M. (2002). Ebstein’s anomaly in adults. The International Journal of Cardiovascular Imaging, 18(6), 439-441. | |||
#Mulder11 Mulder, B. J. M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Ebstein anomaly of the tricuspid valve. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum. | |||
#Beghetti1 Beghetti, Maurice, & Tissot, C. (2009). Pulmonary arterial hypertension in congenital heart diseases. Seminars in Respiratory and Critical Care Medicine, 30(4), 421-428. | |||
#Beghetti2 Beghetti, Maurice, & Tissot, C. (2010). Pulmonary hypertension in congenital shunts. Revista Española De Cardiología, 63(10), 1179-1193. | |||
#Diller Duffels, M G J, Engelfriet, P. M., Berger, R. M. F., van Loon, R. L. E., Hoendermis, E., Vriend, J. W. J., van der Velde, E. T., e.a. (2007). Pulmonary arterial hypertension in congenital heart disease: an epidemiologic perspective from a Dutch registry. International Journal of Cardiology, 120(2), 198-204. | |||
#Engelfriet3 Engelfriet, Peter M, Duffels, M. G. J., Möller, T., Boersma, E., Tijssen, J. G. P., Thaulow, E., Gatzoulis, M. A., e.a. (2007). Pulmonary arterial hypertension in adults born with a heart septal defect: the Euro Heart Survey on adult congenital heart disease. Heart (British Cardiac Society), 93(6), 682-687. | |||
#Galie Galie, N., Hoeper, M. M., Humbert, M., Torbicki, A., Vachiery, J.-L., Barbera, J. A., Beghetti, M., e.a. (2009). Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). European Heart Journal, 30, 2493-2537. | |||
#Gatzoulis Gatzoulis, M A, Alonso-Gonzalez, R., & Beghetti, M. (2009). Pulmonary arterial hypertension in paediatric and adult patients with congenital heart disease. European Respiratory Review: An Official Journal of the European Respiratory Society, 18(113), 154-161. | |||
#Lau Lau, E. M. T., Manes, A., Celermajer, D. S., & Galiè, N. (2011). Early detection of pulmonary vascular disease in pulmonary arterial hypertension: time to move forward. European Heart Journal, 32(20), 2489-2498. | |||
#Mulder12 Mulder, B J M. (2010). Changing demographics of pulmonary arterial hypertension in congenital heart disease. European Respiratory Review: An Official Journal of the European Respiratory Society, 19(118), 308-313. doi:10.1183/09059180.00007910 | |||
#Mulder13 Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Eisenmenger syndrome. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum. | |||
#Schuuring2 Schuuring, M J, van Riel, A. C. M. J., Bouma, B. J., & Mulder, B. J. M. (2011). Recent progress in treatment of pulmonary arterial hypertension due to congenital heart disease. Netherlands Heart Journal: Monthly Journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation, 19(12), 495-497. | |||
#Schuuring Schuuring, Mark J, Vis, J. C., Duffels, M. G., Bouma, B. J., & Mulder, B. J. (2010). Adult patients with pulmonary arterial hypertension due to congenital heart disease: a review on advanced medical treatment with bosentan. Therapeutics and Clinical Risk Management, 6, 359-366. | |||
#Simonneau Simonneau, G., Robbins, I. M., Beghetti, M., Channick, R. N., Delcroix, M., Denton, C. P., Elliott, C. G., e.a. (2009). Updated clinical classification of pulmonary hypertension. Journal of the American College of Cardiology, 54(1 Suppl), S43-54. | |||
#Vis Vis, J. C., Duffels, M. G., Mulder, P., de Bruin-Bon, R. H. A. C. M., Bouma, B. J., Berger, R. M. F., Hoendermis, E. S., e.a. (2011). Prolonged beneficial effect of bosentan treatment and 4-year survival rates in adult patients with pulmonary arterial hypertension associated with congenital heart disease. International Journal of Cardiology. | |||
</biblio> | |||
Latest revision as of 16:52, 1 February 2012
Septal defects
Atrial septal defect
Case Report
Introduction
Atrial septal defect (ASD) is common, accounting for approximately 7 percent of congenital heart disease. The ASD’s can occur in several different anatomic portions of the atrial septum, and the location of the defect generally reflects the abnormality of embryogenesis that led to the anomaly. The functional consequences of an ASD are determined by its diameter, the anatomic location and the presence or absence of other cardiac anomalies.
Classification
The various forms of ASD’s are differentiated from each other by the structures of the heart involved and the formation during embryological development.
The ostium secundum defect is the most frequent form of ASD (70%), localized at the fossa ovalis with a diameter of about 1 – 2 cm. It commonly arises from an enlarged foramen ovale, inadequate growth of the septum secundum or excessive absorption of the septum primum.
The sinus venosus defect (15% of all ASD’s) is localized high in the atrial septum, at the inflow of the superior caval vein. Note that in 80-90% of patients this defect is accompanied by an anomalous pulmonary venous drainage of the right pulmonary vein into the right atrium. Inferior sinus venosus defects do exist, but are very exceptional.
The ostium primum defect is localized low in the atrial septum at the atrioventricular junction. It forms the atrial component of the category of congenital heart disease referred to as atrioventricular defects, with a common atrioventricular junction and an abnormal atrioventricular valve.
The coronary sinus defect, localized at the atrial ostium of the coronary sinus, is rare and usually accompanied by other cardial defects like anomalous drainage of the superior vena cava.
Pathophysiology
The presence of an ASD will in all cases gradually lead to a left to right shunt across the defect. At birth the volume of blood shunting from systemic to pulmonary circulation is small, because the right ventricle is still relatively thick-walled and noncompliant. In response to a decrease in pulmonary vascular resistance after birth, the right ventricle remodels and its compliance increases. This leads to a decrease in right atrial pressure and an increase in shunt volume across the defect during the first years of life.
The blood shunts during the late systole and early diastole, leading to a diastolic volume overload of the right atrium and right ventricle, but also the pulmonary veins and arteries. This volume overload of the pulmonary circulation will consequently lead to right-sided dilatation. The end diastolic increase in pressure of pulmonary circulation can result in systemic venous stuwing. This stuwing is augmented by another mechanism caused by the right ventricular volume overload; deviation of the ventricular septum to the left and the decrease in left ventricle preload because of the left to right shunt, lead to a decrease in stroke volume of the left ventricle. The renine-angiotensin system is activated, leading to an increase in intravascular volume and signs of venous stuwing. The right-sided volume overload is usually well tolerated for years, but in adulthood hemodynamic factors can influence the shunt size in both directions. If the right ventricle will start failing due to chronic volume overlad, the left to right shunt can decrease. If the left ventricle function will decline due to hypertension or coronary artery disease, the lef to right shunt can increase. In 10-20% of adult patients with an isolated ASD pulmonary hypertension will develop, leading to a decrease in left to right shunt and eventually right to left shunt with cyanosis (Eisenmenger syndrome).
Evaluation and therapy
Most ASDs less than 8mm in diameter close spontaneously in infants, however above the age of 4 years spontaneous closure is unusual. During childhood and early adulthood most patients with moderate to large uncorrected ASDs are asymptomatic. Most of them will become symptomatic during adulthood (usually from the age of 40) and require closure of the defect. Indications for closure of an ASD in adulthood are development of symptoms and a high rate of shunt flow. Decreased exercise tolerance, fatigue, dyspnoe, syncope and paradoxal embolization are manifestions of such symptomatic ASDs that warrant closure of the defect. Atrial arrhythmias are usually one of the first presenting symptoms, however these symptoms alone are not an indication for closure, since the incidence after the procedure is not likely to be reduced.
When closure of the ASD is indicated, this can be performed with surgery or percutaneous intervention. Surgical closure is usually performed using a patch of pericardium or Dacron. Prior to surgery, a comprehensive noninvasive evaluation is essential to exclude pulmonary hypertension and associated anatomic defects such as anomalous pulmonary and systemic venous connections. In nearly all cases, echocardiography can resolve these questions, obviating the need for cardiac catheterization.Transcatheter closure avoids cardiopulmonary bypass, thoracotomy, and atriotomy, and is associated with excellent outcomes. As a result, this approach has largely replaced surgery in many centers for patients with a defect that is less than 20 mm in diameter.
Outcome
The short- and long-term outcomes are generally excellent after either surgical or transcatheter closure of an isolated ASD in children. Several investigations showed that there is almost no increase in long-term mortality or serious morbidity compared to controls, following surgical repair of an ASD under 25 years of age. The perioperative mortality is low (< 1%) and there are few perioperative complications (about 10%). However late in adulthood about 50% of all patients develop sinusknoopdysfunctie and symptomatic supraventricular tachyarrhythmias.
When the ASD is closed percutaneously the short-term outcomes (less than one year) are excellent, with reported procedure success rates of 88 to 98%.
Ventricular septal defect
Case report
Introduction
The ventricular septal defect is the most common congenital heart defect in childhood (30%). Most patients have an isolated VSD, however a VSD also occurs in combination with other defects like Tetralogy of Fallot, which will be discussed elsewhere. About five percent of all patients with a VSD have a chromosomal abnormality, including trisomy 13, 18 and 21. Due to a high rate of spontaneous closure (50%) VSD is less seen in adulthood.
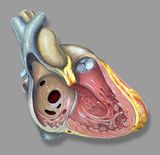
There are three main anatomic components of the interventricular septum (Figure 3); the septum of the atrioventricular canal (1), the muscular septum (2), the parietal band or distal conal septum (3). VSDs may occur at various locations in any of the three components. The location of the defect is not of particular interest when taking the characteristics of the intracardiac shunt in account. However it is important in terms of the frequency of involvement of the atrioventricular valves and the rate of spontaneous closure and additionally the relation to the AV pathway when considering surgical correction.
Classification
The VSD can be classified into four types, related to the anatomic components involved;
- Type 1 is the infundibular VSD, which results from a defect in the septum below the aortic and pulmonary valves. The loss of support to the adjacent septal leaflets of these valves causes cusp prolapse into the VSD leading to progressive aortic regurgitation, which is the hallmark of this defect.
- Type 2, the membranous VSD, is the most common type of VSD (around 80%) and results from a deficiency of the membranous septum. This defect borders the septal leaflet of the tricuspid valve and might also extend into the muscular septum when it is referred to as a perimembranous VSD.
- Type 3 are inlet VSDs, located beneath both mitral and tricuspid valves. Despite proximity to those valves, this type of defect is not associated with mitral or tricuspid regurgitation unless associated with atrioventricular canal defect. This typically large defect is often associated with Down syndrome.
- Muscular defects (type 4) are located within the trabecular septum and accounts for 5 – 20% of all VSDs. It is bordered only by muscle, away from the cardiac valves. Muscular defects can be small or large in size and consist of a single or multiple defects.
Pathophysiology
The severity of the shunt across the VSD is determined by its size and the ratio of pulmonary to systemic vascular resistance. In small or restrictive VSDs the diameter of the defect is ≤25% of the aortic annulus diameter. These small defects cause small left to right shunts with no left ventricular overload or pulmonary hypertension.
Moderate sized VSDs, measuring 25 – 75% of the aortic annulus diameter, result into mild volume overload of the pulmonary arteries, left atrium and left ventricle with no or only mild pulmonary hypertension.
In large defects, defined as those with diameters equal or greater than 75% of the aortic annulus, there is no restriction of blood flow across the septum, leading to equal pressures in both right and left ventricle. The large left to right shunt initially only leads to excessive volume overload in the pulmonary arteries, left atrium and left ventricle. The chronic pressure and volume overload combined with the increase flow leads to irreversible changes of the pulmonary vasculature, which results in an increase in pulmonary vascular resistance. This increase in resistance leads to a reversal of the shunt through the VSD causing right to left shunt with cyanosis (Eisenmenger syndrome).
Evaluation
Small defects are usually asymptomatic, however are fairly easy to detect during physical examination. There can be a palpable thrill accompanied by a holosystolic murmur grade 4-6. In some muscular defects the murmur is non holosystolic, due to the contraction and therby closure of the defect during systole. In moderate sized defects, with a large systemic to pulmonary flow of at least 1:2, a middiastolic rumble is audible due to the increased flow across the mitral valve. The pulmonary component of the second heart tone is loud. Only in a large VSD accompanied by the Eisenmenger syndrome and central cyanosis there is barely any shunt murmur audible. However there are murmurs audible caused by the pulmonary hypertension; an earlydiastolic murmur due to the pulmonary valve regurgitation and a holosystolic murmur due to the tricuspid valve regurgitation. Defects with large shunts that cause symptoms like decreased exercise tolerance and dyspnoe are usually detected and closed early in childhood.
The ECG and chest X-ray of a patient with a small VSD are usually normal, but can show signs of left atrial and left ventricle overload in moderate sized defects.
With echocardiography the localisation, size and hemodynamic influence of the VSD can be investigated. Dilatation of the left atrium and left ventricle might be present and the pressures in the pulmonary artery can be estimated by means of the tricuspid regurgitation. Invasive measurement by means of catheterization is only indicated when there is doubt about the shunt size and the pulmonary vascular resistance.
Treatment
Treatment and prognosis of a VSD depends on the size en localisation of the defect, the pulmonary vascular resistance and possible concomitant defects. Spontaneous closure occurs mainly in small defects, of which 75 percent closes before age 10. In patients with a small defect no pulmonary hypertension develops, however there is an increased risk of endocarditis.
In patients with significant shunts studies have shown that surgical closure reduces pulmonary artery pressure and improves long-term survival. Therefore, repair of VSD should be considered in all adult patients who are symptomatic or have signs of left ventricular volume overload.
Medical treatment is reserved for (1) asymptomatic patients without evidence of left ventricular volume overload and (2) patients with symptoms and/or left ventricular volume overload who are not candidates for repair such as those with large defects and Eisenmenger syndrome.
Repair of VSD has been historically performed surgically. However, percutaneous VSD repair has been growing given the desire of young adults to avoid surgery. Surgical and percutaneous VSD closure should be performed by surgeons and cardiologists with appropriate training and expertise.
Indications for closure of a VSD in an adult are included in the 2008 American College of Cardiology/American Heart Association (ACC/AHA) adult congenital heart disease guidelines as follows. Similar recommendations are included in the European Society of Cardiology and the Canadian Society of Cardiology guidelines.
- Closure of a VSD is indicated when there is a Qp/Qs ≥2 and clinical evidence of LV volume overload.
- Closure of a VSD is indicated when the patient has a history of infective endocarditis.
- Closure of a VSD is reasonable when there is net left to right shunting with a Qp/Qs ≥1.5 with pulmonary artery pressure less than two thirds of systemic pressure and PVR is less than two thirds of systemic vascular resistance.
- Closure of a VSD is reasonable when there is net left to right shunting with a Qp/Qs ≥1.5 in the presence of LV systolic or diastolic dysfunction or failure.
Indications for surgical closure of VSD in infants and young children may include:
- Infants <6 months (<3 months for those with trisomy 21) who have uncontrolled heart failure despite maximal medical and dietary interventions or who have pulmonary hypertension.
- Children with a persistent significant shunt (Qp:Qs >2:1), should undergo surgical repair even in the absence of elevated PA pressures.
- Subpulmonic and membranous defects, regardless of size, with aortic regurgitation should be surgically corrected before the aortic valve is permanently damaged. The decision to close small defects with aortic valve prolapse without aortic regurgitation is controversial.
Closure of a VSD is not recommended in patients with severe irreversible pulmonary artery hypertension.
Transcatheter device VSD closure is a treatment option for isolated uncomplicated muscular VSDs, and for certain membranous VSDs, in selected patients with suitable anatomy. Appropriate anatomy for transcatheter closure includes a VSD location remote from the tricuspid and aortic valves with an adequate rim. Successful transcatheter closure has been accomplished in the presence of multiple muscular or membranous fenestrations.
The technical success rate of transcatheter closure of selected muscular and membranous VSDs is high (93-100%) and the mortality rate is low (0 – 2.7%).
Outcome
The long-term outcome for children who undergo surgical closure of VSD in childhood is generally excellent. Early surgical repair results in near normal long-term growth in the vast majority of patients. Most survivors are asymptomatic and lead normal lives.
Adults with repaired VSD without residua have excellent outcomes and normal survival, with a 25year survival of 83%. However, long-term survival is less favorable when repair is done at older age or in presence of pulmonary hypertension. Although the prognosis after surgical repair of uncomplicated VSD is excellent in the majority of patients, long-term residua and sequelae are not uncommon including conduction disease, cardiac arrhythmias, residual VSD, endocarditis, tricuspid regurgitation, ventricular dysfunction, pulmonary hypertension, and aortic regurgitation.
Development of complete atrioventricular block is the most significant of the transcatheter procedural complications. Approximately 6% of patients who underwent transcatheter closure of membranous defects, developed complete heart block necessitating pacemaker implantation. Real-time three-dimensional transesophageal echocardiography has been increasingly used to guide such procedures. Given the lack of data on long-term outcomes following catheter closure of VSD in adults, patients should be followed every one to two years at an adult congenital heart disease center.
Atrioventricular septal defect
Case report
Introduction
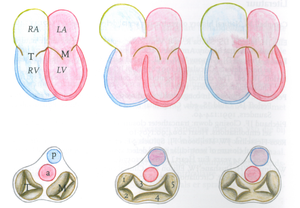
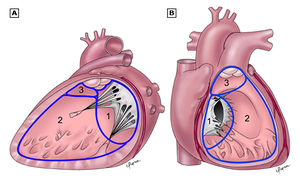
The atrioventricular septal defect (AVSD) consist of several different lesions with a common atrioventricular (AV) junction and abnormal AV valves, consisting of five leaflets (Figure 5). The AVSD makes 3 procent of all congenital heart defects in children.
When the AVSD is complete it consists of a defect on the atrial and on the ventricular side of the common AV-valve ring. (Figure 5, middle). The complete AVSD is usually associated with Down Syndrome, but also with other cardiac defects like ASD type 2, persisting left inferior caval vein and tetralogy of Fallot.
In an incomplete AVSD the superior and inferior bridging leaflets are connected with each other and with the interventricular septum in the centre. (Figure 5, right) Due to this connection there are two divided AV inlets, leaving no open communication between the ventricles, thus no VSD exists. However there is a rather large defect in the interatrial septum. The incomplete AVSD is often referred to as ostium primum defect or ASD type 1.
The left AV-valve consist of three leaflets (there is a cleft in the mitral valve) and is usually incompetent. Due to one common AV junction in both types of AVSD, the aortic valve is not in the usual wedged position between the two separate AV inlets, but located more anterior. Therefore the outflow tract of the left ventricle is elongated and slightly constricted. In angiography this abnormally shaped outflow tract is known as a gooseneck.
Pathophysiology
The exact pathophysiology depends on the location and severity of the defect. In a complete AVSD there is combined right and left ventricular overload due to the left-to-right shunt at atrial and ventricular level combined with the regurgitation of the right and left AV valve. As a result the elevated pulmonary pressures due to the extremely high flow will rapidly convert to pulmonary hypertension. This will lead to bidirectional shunting across the defect with a preferably right-to-left shunt and cyanosis (Eisenmenger syndrome).
In an incomplete AVSD the hemodynamic consequences are comparable to an ASD type 2. However the serious left AV-valve regurgitation can cause an increase in the atrial left-to right shunt.
Evaluation
The complete AVSD leads to symptoms of heart failure early in childhood and most patients will have been surgically corrected in adulthood. During physical examination signs of a residual shunt or regurgitation of the AV-valves might be present. This defect is rarely diagnosed in adulthood and usually there is Eisenmenger syndrome present, with clinical signs previously described in isolated ASD or VSD. The clinical symptoms of an incomplete AVSD are comparable with those of an ASD type 2. During physical examination a murmur of the regurgitant AV-valve might be audible.
The ECG shows a deviation of the heart axis to the left, in contrast to the right axis deviation in atrial septal defects. This is due to the abnormal position of the His bundle which causes a delay in conduction through the left anterior fascicular branch.
Treatment and outcome
The survival for patients with a complete AVSD without corrective surgery is very limited, most patients will not reach adulthood. Therefore surgical correction at an early age (usually in the first year of life) is advised. Patients with a corrected complete AVSD need lifelong cardiologic follow up, due to frequent residual defects like residual VSD, progressive regurgitation of the left AV-valve, pulmonary hypertension and often rhythm- or conduction disorders. Patients with Down Syndrome and complete AVSD are more likely to develop progressive pulmonary hypertension, even after corrective surgery.
The aberrant anatomy of the AV-valves, even after corrective surgery, is important when reviewing echocardiographic images. They can, for example, be easily mistaken for vegetations in endocarditis.
The survival for patients with an incomplete AVSD is higher compared to the complete AVSD, but generally worse compared to other ASD types. This is due to the concomitant disorders of the left AV-valve and the conduction system. Left AV-valve regurgitation will lead to an increase in left-to-right shunt and earlier development of pulmonary hypertension compared to ASD type 2. In childhood there is usually already an indication for correction of the defect in which the anatomically abnormal AV-valve can be repaired. However a slight amount of regurgitation will remain present. In some cases the progressive failure of the AV-valve will require a second repair or replacement, but in most patients the insufficiency will remain mild.
Besides the AV-valve problems there are frequent rhythm and conduction disorders; atrial fibrillation, supraventricular tachycardia, complete heartblock or sinus dysfunction. Depending on the kind of disorder patients can require medical treatment or a pacemaker.
Patent ductus arteriosus
Case report
Introduction
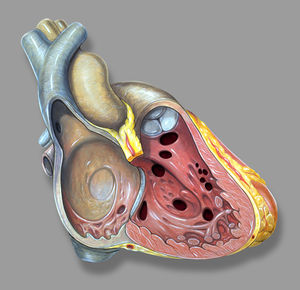
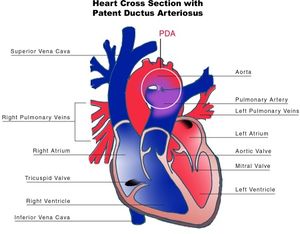
The ductus arteriosus (DA) is a fetal vascular connection between the main pulmonary artery and the descending aorta that diverts blood away from the pulmonary bed (Figure 6). After birth, the DA undergoes active constriction and eventual obliteration. A patent ductus arteriosus (PDA) occurs when the ductus fails to completely close postnatally.
The incidence of PDA has increased dramatically over the last two decades. This is due to the improved survival rate of premature infants, because the incidence of PDA significantly increases in infants born before 30 weeks gestation.
The reported incidence of an isolated PDA among term infants ranges from 0.03 to 0.08 percent.
There is a female predominance for PDA with a 2:1 female to male ratio in most case series of term infants. The incidence of PDA is also greater in infants born at high altitude compared to those born at sea level, and in infants with congenital rubella.
PDA may present with other congenital heart lesions, especially those associated with hypoxemia. PDA should be considered when the clinical features of left-to-right shunt seem out of proportion to the particular lesion being considered.
Evaluation
The clinical manifestations of a PDA are determined by the degree of left-to-right shunting, which is dependent upon the size and length of the PDA, and the difference between pulmonary and systemic vascular resistances.
The hemodynamic consequences of the PDAs can be categorized by the degree of left-to-right shunting based upon the pulmonary to systemic flow ratio (Qp:Qs) [21].
- Small — Qp:Qs <1.5 to 1
- Moderate — Qp:Qs between 1.5 and 2.2 to 1
- Large — Qp:Qs >2.2 to 1
Typical findings during physical examination are a continuous murmur and a low diastolic blood pressure. In small shunts the ECG and chest x-ray are normal. In larger left-to-right shunts signs of left atrial and left ventricular overload might be present.
Echocardiography is a very sensitive and specific method to identify the left-to-right shunt.
Treatment and outcome
Patients with an open PDA have an increased risk of infectious endarteritis, heart failure, pulmonary hypertension and most of these patients become symptomatic in adulthood. Patients with a non-restrictive PDA rarely reach adulthood, unless the pulmonary vascular resistance increases leading to a decrease in left ventricular overload. This hemodynamic state is known as Eisenmenger syndrome in which the shunt is reversed and there is cyanosis present. Patients in who the ductus is closed in childhood have a normal life expectancy.
In patients with a PDA, the primary management decision is whether to actively close the PDA, or to conservatively observe and monitor the patient's cardiac status on a regular basis.
PDA closure is recommended for patients with a significant left-to-right shunt who are symptomatic, have evidence of left-sided volume overload or have reversible pulmonary arterial hypertension. Closure results in resolution of symptoms and a decrease in the likelihood or severity of PAH, and the development of irreversible pulmonary vascular disease (Eisenmenger syndrome).
PDA closure is not recommended in patients with severe and irreversible PAH because of the procedural risk, the fact that closure does not improve survival, and right to left ductal shunting may be necessary to maintain cardiac output during episodes of increasing pulmonary vascular resistance.
Interventions for PDA closure include: pharmacological treatment which is used exclusively in premature infants, surgical ligation or percutaneous catheter occlusion. Surgical closure has a low mortality (<1%) in children and young adults. In adult patients the perioperative risk is increased (around 3%) due to a higher rate of complications like bleeding, heart failure in a compromised left ventricular function and damage to the recurrent laryngeal nerve or phrenic nerve.
Percutaneous PDA occlusion was first introduced in 1967 and provides an alternative to surgical ligation. Many different techniques have been developed, however the two techniques most commonly used are coils or occlusion devices. Both techniques lead to a full occlusion of the PDA and normalization of left ventricular hemodynamics.
Coarctation of the aorta
Case report
Introduction
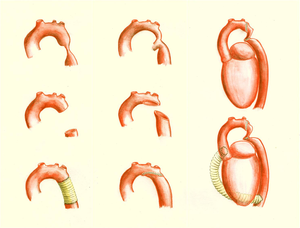
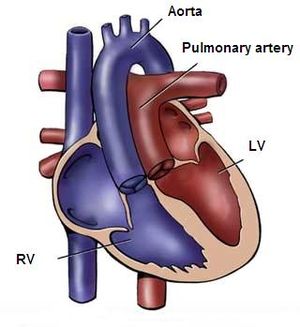
Coarctation of the aorta is a narrowing of the thoracic aorta, typically located in the region of the obliterated ductus arteriosum. (Figure 9) The relation to the position of the left subclavian artery differs, in most patients the left subclavian artery is located anterior of the coarctation. Aortic coarctation is frequently associated with diffuse hypoplasia of the aortic arch and isthmus.
The incidence of coarctation of the aorta is 4 in 10.000 live births, accounting for 5–9% of the children with congenital heart defects, occurring two to five times more frequently in males than females.
Coarctation of the aorta can be an isolated congenital heart defect, however usually it coincides with other congenital defects. Associated heart defects are patent ductus arteriosus, ventricular septal defect, mitral valve stenosis and valvular and subvalvular aortic stenosis. Furthermore around 75% of all patients with a coarctation of the aorta have a bicuspid aortic valve.
The development of coarctation aorta depends on genetic as well on non-genetic factors. Parents with coarctation aorta have a 2% (male) or 4% (female) chance of passing this defect to their child.
Pathophysiology
Coarctation aorta has no hemodynamic consequences in utero, because only 10% of the total cardiac output crosses from the ascending to the descending aorta. However after birth the ductus arteriosus and foramen ovale close, leading the whole cardiac output through the narrowed aortic segment. This leads to an increase in resistance in the left ventricular outflow tract, resulting in an elevated systolic pressure in the left ventricle and upper extremities. When coping with the elevated pressures, the left ventricle will become hypertrophic.
If the coarctation is severe or in the acute phase (after birth when the ductus is closed), systolic dysfunction of the left ventricle and heart failure can occur.
Most adult patients are asymptomatic unless severe hypertension is present leading to headache, epistaxis, heart failure, or aortic dissection. In addition, claudication may occur due to reduced flow to the lower extremities.
Evaluation
Coarctation of the aorta is easily diagnosed without invasive methods, by means of physical examination, echocardiography or MRI. The combination of weak or absent femoral arterial pulses and upper body hypertension in physical examination points into the direction of coarctation of the aorta. Nevertheless, studies have shown that the diagnosis in hypertensive patients is often missed by the referring doctor. As a consequence, a significant number of asymptomatic subjects with aortic coarctation are probably not detected until adult life, so their incidence at birth is likely to be underestimated. Late detection of subjects with aortic coarctation can have detrimental effects on survival. For, without correction, the mean life expectancy of patients with aortic coarctation is 35 years and 90% of those patients die before reaching the age of 50 years.
Chest radiograph varies with age and severity of the coarctation. In infants with heart failure, the chest radiograph shows generalized cardiomegaly with increased pulmonary vascular markings due to pulmonary venous congestion. In older children and adults, the heart size may be normal but notching of the posterior one-third of the third to eighth ribs due to erosion by the large collateral arteries might be present.
Transthoracic echocardiography, including suprasternal notch views, is useful for initial imaging and hemodynamic evaluation in suspected aortic coarctation. Echocardiographic evaluation should also include measurement of the dimensions of the aortic annulus, aortic sinuses, sinotubular ridge, and ascending aorta; identification of aortic valve anatomy; determination of left ventricular size and function; and identification of any potential associated lesions such as ventricular septal defect, subvalvular aortic stenosis and mitral valve deformity.
Treatment and outcome
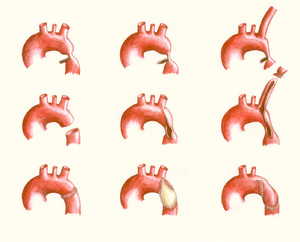
Since surgical repair of aortic coarctation became available in 1944, survival of patients with aortic coarctation has dramatically improved and the number of patients who were operated on and reach adulthood is steadily increasing. However, life expectancy is still not as normal as in unaffected peers. Survival of patients operated at a median age of 16 years was 91% at 10 years, 84% at 20 years and 72% at 30 years after operation. Survival of post-coarctectomy patients is significantly affected by age at operation and nowadays early repair is advocated. Even after early repair—before the age of 5 years—the estimated survival is still reduced, with 91% of the operated patients alive at 20 years and 80% at 40 to 50 years after surgery. However, repair of aortic coarctation is still recommended in patients at older age when diagnosis is delayed, because it improves blood pressure regulation and is probably associated with a lower risk of cardiovascular events in later years and improved survival.
There are several surgical techniques used for correction of the aortic coarctation. (Figure 10 & 11) Resection of the narrowed aortic segment with end-to-end anastomosis is the most commonly used technique. The subclavian flap aortoplasty and dilatation with a patch are not in use anymore due to a decreased blood flow in the left arm and a high rate of aneurysmatic deformation of the vessel respectively. When end-to-end anastomosis is not feasible, an interposition graft might be used instead. Sometimes a complete resection of the stenosis is not possible, for example when the carotid arteries are part of the coarctation, then an extended aortic arch repair or extra-anatomic bypass might be an appropriate choice. (Figure 7)
Transcatheter interventions for native aortic coarctation have been used for over 20 years. Transcatheter treatment for native aortic coarctation has been shown to be feasible, relatively safe and effective at short term and intermediate follow-up and is rapidly becoming the treatment of choice. Older age, however, seems to be a risk factor for suboptimal outcome after balloon angioplasty possibly due to a more fibrotic and rigid aorta. Especially in the full grown patient, stent placement seems a particularly attractive option, resulting in an almost complete relief of the gradient in 95% of the patients. Another benefit of stent placement is the ability to address longer segment coarctations, which typically have a poorer outcome after balloon angioplasty alone. Long-term results, however, are to be awaited. Concern after surgery or catheter intervention falls chiefly in seven categories: recoarctation, aortic aneurysm formation or aortic dissection, coexisting bicuspid aortic valve, endocarditis, premature coronary atherosclerosis, cerebrovascular accidents and systemic hypertension.
Transposition of the great arteries
Case report
Introduction
Transposition of the great arteries (TGA) accounts for 5-8% of all congenital heart defects and occurs 2-3 times more frequently in males. TGA is best defined as a normal atrioventricular connection with an abnormal ventricular–arterial connection; the morphological left atrium is connected through the left ventricle with the pulmonary artery and the morphological right atrium through the right ventricle with the aorta. (Figure 12)The aorta is often located on the right side and in front of the pulmonary artery (D-TGA). In 70 percent there is an isolated form of TGA, in 30 percent the TGA is accompanied by other heart defects, like VSD or obstruction of the left ventricle outflow tract.
Pathophysiology
The circulation in TGA patients is not serial but parallel (Figure 13); the venous blood is returned to the systemic circulation through the right atrium and ventricle, while the arterial oxygenated blood is directed back into the pulmonary artery through the left atrium and ventricle. Due to this abnormal circulation there is severe cyanosis directly after birth, therefore it is critical for the ductus arteriosus and foramen ovale to remain open. Without treatment there is a mortality of 30% within one week, 50% within one month and 90% within one year. When an associated VSD is present the chances of survival are higher due to more shunting thus more oxygenated blood in the systemic circulation. These patients are able to reach early adulthood without corrective surgery or intervention. However the pulmonary hypertension that develops in this situation will eventually lead to severe problems.
Treatment
Mortality of TGA has dramatically improved from 90 percent for unoperated patients to rates of less than 5 percent following corrective surgery using the arterial switch operation. Most patients are referred for surgical repair during the first three to five days of life. The choice of surgical procedure is dependent on the presence and nature of other cardiac anomalies. In patients without any other cardiac defect (simple D-TGA), arterial switch operation is the recommended procedure. In general, the arterial switch operation has replaced the earlier atrial switch procedures developed by Mustard and Senning. In patients with D-TGA and a ventricular septal defect (VSD), the preferred procedure is arterial switch operation and VSD closure. In patients with D-TGA, large VSD, and significant pulmonary stenosis, the Rastelli procedure, an alternative surgical approach, should be considered. In some cases, arterial switch operation with or without repair of the left ventricular outflow obstruction is used. Both the arterial switch operation and Rastelli procedures are surgical anatomic corrections resulting in a morphologic left ventricle as the systemic ventricle. In comparison to atrial switch procedures, which involved the rerouting of venous return in the atria and are now only rarely performed, the arterial switch operation appears to have similar long-term survival rates with reduced long-term morbidity primarily due to a lower risk of atrial arrhythmias and heart failure. As a result, it is the recommended procedure in most patients with D-TGA.
Outcome
The long-term survival rates for patients with D-TGA following surgical correction are excellent for both arterial switch operation and atrial switch procedures. The long-term survival 20 years after discharge is about 95 and 80 percent for arterial switch and atrial switch respectively. Progressive congestive heart failure and sudden death are the principal causes of death. Perioperative mortality is greater in patients with complex (additional cardiac anomaly) D-TGA compared to those with simple D-TGA. There are no clinical trials comparing outcomes of arterial switch and atrial switch procedures. Reintervention is common following both approaches.
Patients who undergo surgical repair for D-TGA have a reduced exercise capacity primarily due to pulmonary disease. Patients after arterial switch operation appear to have better exercise capacity than those who underwent atrial switch procedures. The decrease in exercise capacity, however, does not limit ordinary activity as most patients meet the criteria for New York Heart Association functional class I.
It appears that patients with D-TGA may have mild long-term neurodevelopmental impairment, most likely due to perioperative factors including hypoxemia, acidosis, cardiopulmonary bypass, and hemodynamic instability.
Congenitally corrected transposition of the great arteries
Congenitally corrected transposition of the great arteries
Introduction
The congenitally corrected transposition of the great arteries (ccTGA) is characterized by a normal anatomical position of both atria, with an abnormal connection between the atria and the ventricles. The right atrium is connected with the left ventricle and the left atrium is connected with the right ventricle. (Figure 14) Furthermore the aorta arises from the right ventricle and the pulmonary artery from the left ventricle. There are, in conclusion, abnormal atrioventricular connections and abnormal ventricular-arterial connections present in ccTGA. CcTGA is a very rare defect, accounting for about 1% of all congenital heart disease.
Pathophysiology
The blood flow in ccTGA is ‘corrected’ because oxygenated blood flows through the systemic circulation and deoxygenated blood is transported to the lungs. In this particular heart defect the right ventricle functions as a systemic ventricle. With the atrioventricular valves following the position of the ventricles, the tricuspid valve is now the systemice AV-valve.
Additionally the coronary arteries are a mirror image of the normal anatomic situation. The coronary artery on the right side of the heart runs like a morphological left coronary artery; starting with a main stem which divides into a left anterior descending and circumflex branch. The coronary artery on the left is the morphological right coronary artery, which now runs around the tricuspid valve on the left.
Even the conduction system is abnormal in this congenital heart defect. Normally the AV node is positioned at the base of the interatrial septum, from where the His bundle arises into the interventricular septum. Due to misalignment in ccTGA there is no bundle directly from the normal AV node into the interventricular septum. Usually there is an additional AV node located more anterior and laterally from where a long bundle arises which runs beneath the pulmonary valve leaflets into the interventricular septum. Due to the long route this bundle is rather vulnerable. Where in normal hearts the electrical activation of the ventricles in the septum runs from left to right, in ccTGA it is the exact opposite; the septum is activated from right to left, which is visible on the ECG as an abnormal initial activation.
There is a VSD present in 60% of all ccTGA patients, who will often become symptomatic at younger age. Pulmonary valve stenosis is seen in 30 – 50% of patients, usually in those who already have a VSD as well. Abnormal anatomy of the tricuspid valve (M. Ebstein like) is seen in 25-30% of patients, sometimes accompanied by VSD or pulmonary valve stenosis. More than 80 percent of patients have an abnormal function of the tricuspid valve, which often becomes insufficient.
Evaluation
When only ccTGA is present, without other cardiac defects, the diagnosis is often not recognized until adulthood. However, when there is a VSD and/or pulmonary valve stenosis present, it presents short after birth with cyanosis and heart failure or even a total AV-block.
Adult patients are usually recognized due to an abnormal ECG, abnormal chest radiograph, systolic murmur due to the regurgitation of the systemic AV-valve, occurrence of atrial tachycardia or an AV-block. Reduced exercise capacity might be present.
The ECG is the most typical for ccTGA; left heart axis deviation, septum activation in mirror image (no Q-wave in I, aVL and V6, no R-top in V1) and a rather deep Q-wave in III and aVF. Echocardiography is a good diagnostic tool to review the exact anatomy. The right ventricle positioned on the left side is identified by the typical morphology of the trabeculae. Furthermore the tricuspid valve has a lower insertion compared to the mitral valve.
Treatment and outcome
Patients with ccTGA require lifelong follow up due to the risk of arrhythmias and associated sudden death, increase of pulmonary valve stenosis, increase of tricuspid valve regurgitation, supraventricular tachycardia, heart failure or endocarditis. Due to the complex anatomy and the low incidence of this pathology, this is best acquired at a specialised centre of congenital heart disease.
Arrhythmias need treatment, preferably not with negative inotropic agents, but digoxin and possibly amiodaron are preferred. When presenting with atrial arrhythmias conversion to sinus rhythm is highly important in these patients, who are in need of their ‘atrial kick’.
Many patients with ccTGA require pacemaker placement, often already in childhood or early adulthood. Frequent followup in these patients is required due to the high complication rate; dislocation of the lead which is located in the smooth-walled left ventricle and risk of infection or endocarditis.
Determining the right time to replace the tricuspid valve is difficult in these patients. Preferably this is done just before the right systemic ventricle starts dilating and fails, but it remains an estimation which should be made for each individual patient.
To restore normal blood flow through the ventricles, with the left ventricle functioning as the systemic ventricle, there is an option to perform a double switch operation. During this procedure both atria and both great arteries are switched, meaning a combination of the arterial switch and the atrial switch procedure. From a physiologic point of view this operation is worth considering while the left ventricle will function as systemic ventricle, however it is associated with a high perioperative mortality due to the extent of surgery. Furthermore the left ventricle requires training prior to surgery, to be able to cope with the high arterial pressure after years of low pulmonary pressure. In conclusion the chance of success of this double switch operation is very low in patients above 16 years of age.
Univentricular heart
Case report
Introduction
Around 10 percent of all congenital heart defect patients have just one functioning ventricle. The other ventricle is present, however it is rudimentary or underdeveloped so it can not function normally. In utero this causes rarely any problems, due to the parallel circulation the other ventricle takes over both functions. It is after birth, if the ductus arteriosus closes, when problems arise.
Pathophysiology
The hypoplastic left heart syndrome (HLHS) is the most common type of univentricular heart. (Figure 15) Not only the left ventricle, but often the aortic valve, ascending aorta and aortic arch are hypoplastic as well. This will redirect blood from the left atrium into the right atrium, where is will be mixed with venous blood and pumped into the right ventricle and pulmonary artery. The whole systemic circulation depends on the shunt from pulmonary artery through the ductus arteriosus into the aorta. When the ductus starts closing the consequences are dramatic, with severe cyanosis and acidosis.
When a hypoplastic right ventricle is present with associated atresia of the pulmonary artery, the pulmonary circulation after birth will solely depend on the left-to-right shunt through the ductus arteriosus.When the ductus starts closing, progressive cyanosis is the main presenting symptom.
Other cardiac defects associated with only one functional ventricle are: tricuspid valve atresia, mitral valve atresia, severe form of Ebstein anomaly, double inlet left ventricle and unbalanced AVSD.
All patients with one functioning ventricle have complete mixing of saturated and desaturated blood leading to chronic hypoxemia (Figure 24). Furthermore there is a chronic volume overload to the ventricle, serving as both pulmonary and systemic ventricle, leading to an early development of heart failure.
Due to the obligatory intracardiac shunting the pulmonary ‘filter’ is bypassed, which will increase the chance of cardiovascular accidents and brain abscesses.
Treatment
In case of a ductus-dependent defect initial treatment immediately after birth consists of prevention of ductus closure. At first this can be achieved pharmacologically with prostaglandin, however due to the many side effects this is no long-term solution. When there is a dependent pulmonary circulation an aortopulmonary shunt will be constructed during the first weeks of life to ensure accurate blood flow to the lungs after discontinuation of the prostaglandin.
If there is a dependent systemic circulation the surgical treatment usually consists of three different steps. Since the anatomy is by no means normalized, one can not speak of a surgical correction, it is referred to as a definitive palliation. At first a Norwood or Sano procedure is performed in neonates where a neo-aorta is constructed by dividing the pulmonary artery. Second stage is the construction of a cavopulmonary shunt, also known as bidirectional Glenn shunt, which is performed at 4 -6 months of age. The third and final stage is known as Fontan procedure and performed at 18 – 30 months of age, where a total cavopulmonary connection is created. (Figure 16) All surgical procedures are described in more detail separately.
Outcome
With an expanding cohort of survivors of surgical palliation through Fontan completion, increasing information is being accumulated on the long-term morbidity of these patients. Active areas of interest include exercise tolerance, neurodevelopmental outcome, and quality of life.
When assessed prospectively by formal exercise testing, children with HLHS after surgical repair showed considerable age-related decline in exercise performance. Among patients participating in treadmill or bicycle ergometry, those aged 8 to 12 performed at 70 percent of predicted peak oxygen consumption, whereas older children reached only 60 percent of predicted performance.
Several reports have demonstrated significant neurodevelopmental impairment in survivors of HLHS following staged repairs or cardiac transplantation. There is a paucity of data on the quality of life for patients with HLHS. In one report of survivors and their families, parents reported poorer functional health status than patients assessed at 18 years of age.
Risk factors that lower survival include noncardiac congenital anomalies and/or genetic disorder, particularly chromosomal defects, prematurity, low birth weight for gestational age, and living in a high poverty neighborhood.
With an expanding cohort of survivors of surgical palliation through Fontan completion, increasing information is being accumulated on the long-term morbidity of these patients. Active areas of interest include exercise tolerance, neurodevelopmental outcome, and quality of life.
When assessed prospectively by formal exercise testing, children with HLHS after surgical repair showed considerable age-related decline in exercise performance. Among patients participating in treadmill or bicycle ergometry, those aged 8 to 12 performed at 70 percent of predicted peak oxygen consumption, whereas older children reached only 60 percent of predicted performance.
Several reports have demonstrated significant neurodevelopmental impairment in survivors of HLHS following staged repairs or cardiac transplantation.
There is a paucity of data on the quality of life for patients with HLHS. In one report of survivors and their families, parents reported poorer functional health status than patients assessed at 18 years of age.
Risk factors that lower survival include noncardiac congenital anomalies and/or genetic disorder, particularly chromosomal defects, prematurity, low birth weight for gestational age, and living in a high poverty neighborhood.
Tetralogy of Fallot
Case report
Introduction
In 1888 Etienne Louis Arthur Fallot described the ‘maladie bleue’ as a combination of:
- Stenosis of the pulmonary artery (PS)
- Ventricular septal defect (VSD)
- Deviation of the origin of the aorta to the right
- Hypertrophic right ventricle
This constellation of findings has since become known as tetralogy of Fallot (TOF). (Figure 17)
The prevalence of TOF is about 3.9 per 10.000 live births. This defect accounts for about 7 to 10 percent of cases of congenital heart disease and is one of the most common congenital heart lesions requiring intervention in the first year of life.
Anatomy
In fact there is only one anatomic abnormality in TOF; a misalignment of the interventricular septum. As a consequence the muscular part of the interventricular septum is not able to fuse with the cranial part of the septum (leaving a ventricular septal defect) and overriding of the aorta, which causes stenosis of the right ventricular outflow tract. The hypertrophy of the right ventricle is a direct consequence of the elevated pressure in the right ventricle due to the large unrestrictive VSD.
Pathophysiology
The combination of infundibular PS and a VSD causes the blood flow in utero to flow directly into the aorta, leading to a high chance of underdevelopment of the pulmonary valve and arteries. Therefore pulmonary valve stenosis and hypoplasia of the pulmonary arteries are often found in TOF patients. Stenosis at the origin or more distally of the right or left pulmonary artery are frequently found, so is total absence of (usually the left) pulmonary artery. Furthermore about 33% percent of all TOF patients has a descending aorta on the right side.
The physiologic consequences of TOF are largely dependent upon the degree of right ventricular outflow obstruction. Since the VSD is typically large and unrestrictive, the pressure in the right ventricle reflects that of the left ventricle. As a result, the direction of blood flow across the VSD will be determined by the path of least resistance for blood flow, not by the size of the VSD. If the resistance to blood flow across the obstructed right ventricular outflow tract is less than the resistance to flow out of the aorta into the systemic circulation, blood will naturally shunt from the left ventricle to the right ventricle and into the pulmonary bed. In this situation, there is predominately a left-to-right shunt and the patient will be acyanotic.
As the degree of right ventricular outflow obstruction increases, the resistance to blood flow into the pulmonary bed also increases. If the right ventricular obstruction is significant enough to increase resistance, it will be easier for blood to cross the VSD from the right ventricle into the left ventricle and go out the aorta, which now becomes the path of least resistance. This right-to-left shunt across the VSD will result in a large volume of desaturated blood entering the systemic circulation and cyanosis and polycythemia will ensue.
One of the physiologic characteristics of TOF is that the right ventricular outflow obstruction can fluctuate. An individual with minimal cyanosis can develop a dynamic increase in right ventricular outflow tract obstruction with a subsequent increase in right-to-left shunt and the development of cyanosis. In the most dramatic situation, there can be near occlusion of the right ventricular outflow tract (RVOT) with profound cyanosis. These episodes are often referred to as "hypercyanotic spells". The exact etiology of these episodes is unclear, although there have been a number of proposed mechanisms, including increased infundibular contractility, peripheral vasodilatation, hyperventilation, and stimulation of right ventricular mechanoreceptors.
Treatment
Patients with TOF can undergo either palliative (shunts) or corrective (intracardiac repair) surgery. Although most children with TOF undergo intracardiac repair as their initial intervention, the principle of shunts remains an important palliative procedure for infants who may not be acceptable candidates for intracardiac repair due to prematurity, hypoplastic pulmonary arteries, or coronary artery anatomy.
Shunts are constructed to increase the blood flow to the lungs, to improve the development of the pulmonary arteries. Many patients who underwent intracardiac repair initially had a palliative shunt. Blalock and Taussig first reported successful surgical palliation of TOF in 1945. The procedure, which has since come to bear their names, used a subclavian artery to create an aorta-to-pulmonary artery connection. The technique has been modified and is now usually performed using a Gortex tube to create the connection.
A different type of shunt is the aortopulmonary anastomis, where a direct connection between the descending aorta and left pulmonary artery (Potts) or between the ascending aorta and the right pulmonary artery (Waterston) is constructed.
Intracardiac repair of TOF was reported by Lillehi in 1954. It consists of patch closure of the ventricular septal defect and enlargement of the RVOT. The latter is accomplished by relieving pulmonary stenosis, resecting infundibular and subinfundibular muscle bundles and if necessary by a transannular patch, creating unobstructed flow from the RV into the pulmonary arteries.
Outcome
A few decades ago the perioperative mortality in TOF patients was rather high (until 25 percent) but gradually declined to the current risk of around 3%. The survival in TOF patients after surgery is, although slightly less than the average population, considerably good. The longest follow-up cohort shows a survival rate of 85% after 35 years. However the rate of morbidity is very high in TOF patients due to a wide range of residual defects, that can increase over time. Therefore all patients with TOF require a lifelong, regular cardiologic follow up.
The main complications of TOF include pulmonary regurgitation, residual right ventricular outflow tract obstruction, pulmonary hypertension and residual shunt.
Intracardiac repair with a transannular RVOT patch can result in chronic severe pulmonary regurgitation. This leads to RV enlargement and patients may develop decreased exercise tolerance, right heart failure, and arrhythmias. A surgical prosthetic pulmonary valve may be necessary to restore the valve competence and improve RV function and functional status in TOF patients. Residual RVOT obstruction can persist after the original intracardiac operation due to hypertrophied subvalvar muscle, annular hypoplasia, pulmonary valve stenosis, supravalvar pulmonary stenosis, or branch pulmonary artery stenosis. Mild obstruction is usually well tolerated, but significant obstruction may require reoperation or catheter-based intervention. Relief of pulmonary artery stenosis by balloon dilation or stenting may be necessary prior to pulmonary valve replacement.
Pulmonary hypertension can be present in TOF patients due to: hypoplastic pulmonary arteries with associated high vascular resistance, excessive shunting across the surgically constructed shunts (mainly Potts or Waterston shunts) or presence of multiple pulmonary artery stenosis.
A residual VSD shunt is present in about 20 percent of all operated TOF patients, requiring a reoperation in 5 – 10 percent of them. Residual shunt might be due to detachment of the patch or an additional septal defect which was not recognized during surgery.
Marfan syndrome
Case report
Introduction
Marfan syndrome (MFS) is an autosomal dominant condition with a reported incidence of 1 in 3000 to 5000 individuals and is one of the most common inherited disorders of connective tissue. While most MFS patients have an affected parent, around 15 – 30 percent have a de novo mutation. MFS is associated with a broad range of clinical symptoms and associated disorders, ranging from classic ocular, cardiovascular, and musculoskeletal abnormalities to manifestations including involvement of the lung, skin, and central nervous system.
Progressive dilatation of the ascending aorta is one of the key features, which causes a high risk of sudden death due to aortic dissection or rupture in young Marfan patients. (Figure 18 & 19)
The underlying genetic defect is localised in the fibrillin gene on chromosome 15 (FBN1) in which recently around 600 different mutations are found. However in about 10% of MFS patients there is no mutation identified in the FBN1 gene, furthermore FBN1 mutations also occur across a wide range of milder phenotypes that overlap the classic Marfan phenotype. Therefore it is not possible to diagnose MFS solely with genetic information.
Criteria for diagnosis of Marfan syndrome
The 2010 revised Ghent criteria puts greater weight on aortic root aneurysm/dissection and ectopia lentis as the cardinal clinical features of MFS and on testing for mutations in FBN1 and other relevant genes.
In the absence of family history of MFS, the presence of one of any of the following criteria is diagnostic for MFS:
- Aortic criterion (aortic diameter Z≥2 or aortic root dissection) and ectopia lentis*
- Aortic criterion (aortic diameter Z≥2 or aortic root dissection) and a causal FBN1 mutation
- Aortic criterion (aortic diameter Z≥2 or aortic root dissection) and a systemic score ≥7
- Ectopia lentis and a causal FBN1 mutation as defined above that has been identified in an individual with aortic aneurysm
In the presence of family history of MFS (as defined by the above criteria), the presence of one of any of the following criteria is diagnostic for MFS:
- Ectopia lentis
- Systemic score ≥7 points*
- Aortic criterion (aortic diameter Z≥2 above 20 years old, Z≥3 below 20 years, or aortic root dissection)*
For criteria with an asterisk (*), the diagnosis of MFS can be made only in the absence of discriminating features of Shprintzen-Goldberg syndrome (SGS), Loeys-Dietz syndrome (LDS), or vascular Ehlers-Danlos syndrome (vEDS) and after TGFBR1/2, collagen biochemistry, or COL3A1 testing if indicated.
The revised Ghent nosology includes the following scoring system for systemic features:
- Wrist AND thumb sign: 3 points (wrist OR thumb sign: 1 point)
- Pectus carinatum deformity: 2 (pectus excavatum or chest asymmetry: 1 point)
- Hindfoot deformity: 2 points (plain pes planus:1 point)
- Pneumothorax: 2 points
- Dural ectasia: 2 points
- Protrusio acetabuli: 2 points
- Reduced upper segment/lower segment ratio (US/LS) AND increased arm span/height AND no severe scoliosis: 1 point
- Scoliosis or thoracolumbar kyphosis: 1 point
- Reduced elbow extension (≤170 degrees with full extension): 1 point
- Facial features (at least 3 of the following 5 features: dolichocephaly [reduced cephalic index or head width/length ratio], enophthalmos, downslanting palpebral fissures, malar hypoplasia, retrognathia): 1 point
- Skin striae: 1 point
- Myopia >3 diopters: 1 point
- Mitral valve prolapse (all types): 1 point
A systemic score ≥7 indicates systemic involvement.
Pathophysiology
Aortic root disease, leading to aneurysmal dilatation, aortic regurgitation, and dissection, is the main cause of morbidity and mortality in the MFS. Dilatation of the aorta is found in approximately 50 percent of children with MFS and progresses with time. Approximately 60 to 80 percent of adult patients with MFS have dilatation of the aortic root (with normal range adjusted for patient body surface area and age) by echocardiography, often accompanied by aortic regurgitation. Dilatation may also involve other segments of the thoracic aorta, the abdominal aorta, the root of the pulmonary artery or even the carotid and intracranial arteries. Untreated MFS is frequently associated with aortic dissection, which begins just above the coronary ostia and extends the entire length of the aorta; it is a type I dissection in the DeBakey classification or a type A in the Dailey scheme. Approximately 10 percent of dissections begin distal to the left subclavian (type III or type B) but dissection is rarely limited to just the abdominal aorta. Many patients with MFS and aortic dissection have a family history of dissection.
Mitral valve prolapse (MVP) is frequently identified in patients with MFS. However, only one point in the systemic score is assigned for MVP since it is a nonspecific feature and most patients with mitral valve prolapse do not have MFS. The frequency of MVP in MFS increases with age and is greater in women. Tricuspid valve prolapse may also occur.
Treatment
Beta blockers decrease myocardial contractility and may also improve the elastic properties of the aorta, particularly in patients with an aortic root diameter <40 mm thereby decreasing the risk of aortic dissection and delaying the aortic dilatation. Prophylactic treatment with beta blockers is considered the standard of care in adults with MFS. Furthermore patients with MFS are advised to avoid any contact sports, exercise at maximal capacity, and isometric activities.
The exact aortic root diameter at which elective surgery should be performed is uncertain. The current guidelines recommend elective operation for patients with MFS at an external diameter of ≥50 mm to avoid acute dissection or rupture. Indications for repair at an external diameter less than 50 mm include rapid growth (>2 mm/y), family history of aortic dissection at a diameter less than 50 mm, desire of pregnancy or presence of progressive aortic or mitral valve regurgitation. However one must take into account that a predicted aortic root diameter varies with body size and age and may be smaller in women. Smaller patients have dissection at a smaller aortic root size and 15 percent of patients with MFS have dissection at a diameter less than 50 mm.
The classic aortic root surgery is the Bentall procedure in which the ascending aorta is replaced, together with the aortic valve, by a graft with prosthetic valve. In this procedure the coronary arteries need to be reimplanted in the aortic graft. In patients with anatomically normal valves, in whom the insufficiency is due to the dilated annulus or dissection, valve-sparing operations with root replacement by a Dacron prosthesis and with reimplantation of the coronary arteries into the prosthesis (David’s procedure) or remodelling of the aortic root (Yacoub’s procedure) have now become the preferred surgical procedures. Aortic regurgitation is, however, a common complication, requiring reoperation in 20% of patients after 10 years.
Outcome
The reported operative mortality of the Bentall procedure is 1.5% for elective and 11.7% for emergency operations. Five- and 10-year survival rates of 84 and 75%, respectively, have been reported. This relatively limited prognosis is due to late sequelae associated with MFS; 75% require reoperations, 10% mitral valve surgery, 1% develop endocarditis and 1% a CVA. Marfan syndrome has also been associated with a considerably higher risk of re-dissection and recurrent aneurysm than other aetiologies of aortic disease. Long-term results of valvesparing aortic root replacement in Marfan syndrome are still unknown.
Ebsteins anomaly
Case report
Introduction
Ebsteins anomaly, named after Wilhelm Ebstein (1836 – 1912) (Figure 20) is a congenital heart defect of the morphological tricuspid valve. The prevalence of Ebstein's anomaly is about 1 in 50.000 – 200.000 with a similar incidence in both males and females.
As its name clearly indicates, the tricuspid valve consists of three leaflets; anterosuperior, septal and inferior. The Ebstein’s anomaly consists of a variety of anatomical and functional abnormalities of the tricuspid valve. Typical features are:
- displacement of the septal and inferior leaflet downwards from the atrioventricular junction and toward the body of the right ventricle or the apex
- the anterior leaflet is not displaced, enlarged (sail-like) and can show fenestrations
- the tricuspid valve inlet is displaced towards the right ventricular outflow tract and often stenotic
- atrialisation of the right ventricle because of a downward extension of the tricuspid valve, leaving a small right ventricle or only a right ventricular outflow tract
Pathophysiology
The Ebstein’s anomaly can be isolated or associated with other cardiac defects like ASD or patent foramen ovale, pulmonary outflow tract obstruction, VSD, coarctation of the aorta, ccTGA, one or more accessory conduction pathways or patent ductus arteriosus.
The primary hemodynamic consequence of Ebstein’s anomaly is tricuspid regurgitation (TR), which varies in severity. Severe TR causes a volume overload and right-sided cardiac chamber dilation and dysfunction, leading to a decrease in cardiac output. In some cases even hepatic congestion and failure can arise.
Ebstein's anomaly can be classified as mild, moderate, or severe based upon the extent of apical displacement of the valve leaflets with resultant tricuspid regurgitation, and the degree of right ventricular dysfunction.
Symptoms such as cyanosis and heart failure from severe TR may appear soon after birth, because of high pulmonary vascular resistance. However, symptoms often improve as pulmonary vascular resistance decreases. At a later age, symptoms such as exertional dyspnea, fatigue, cyanosis, and palpitations may recur; these symptoms may be insidious in onset. Palpitations due to atrial tachyarrhythmia are present in 20 to 30 percent of cases. Some of these arrhythmias may be due to Wolff-Parkinson-White syndrome, since up to 20 percent of patients have one or more accessory pathways; the majority of these pathways are located around the orifice of the malformed tricuspid valve. Patients with Ebstein's anomaly who have an interatrial communication are at risk for paradoxical embolization, brain abscesses, and sudden death.
Treatment
Indications for surgical tricuspid valve repair or replacement are:
- decrease in exercise capacity
- progressive cyanosis
- progressive right ventricular dilatation or dysfunction
- occurrence of paradoxal embolism
- supraventricular arrhythmias despite pharmacological or ablation therapy
- progressive cardiomegaly on chest radiograph
There are two main surgical techniques used in tricuspid valve repair. The Danielson repair consists of horizontally plicating the atrialized portion of the RV while multiple commissuroplasty stitches are placed. The Carpentier technique consists of detaching the large sail-like anterior leaflet from the valve annulus and translocating it in a clockwise division in order to create what essentially becomes a single leaflet valve. The atrialized portion of the RV is plicated but in a vertical not a horizontal fashion. If repair is not possible and the patient has reached adult size, tricuspid valve replacement becomes necessary. A biologic prosthesis, such as a porcine valve, is usually chosen because of the high incidence of thromboembolism with a mechanical prosthesis placed in the right heart.
Outcome
The prognosis varies with the severity of the disease. The 1 and 10 year survival rates for all liveborn patients have been estimated 67 percent and 59 percent respectively. The major causes of death are heart failure, perioperative, and sudden death.
However, survival is probably increasing as advances in diagnostic and surgical techniques and postoperative care have led to improvements in surgical outcome.
Pulmonary hypertension
Case report
Introduction
Pulmonary hypertension (PH) is a progressive life-threatening condition and associated with a high rate of mortality, despite medical intervention. PH is characterized by elevated pulmonary arterial pressure and secondary right ventricular failure.
The definition of pulmonary hypertension (PH) is based upon right heart catheterization where PH is defined as a mean pulmonary artery pressure greater than 25 mmHg at rest.
Classification
PH is classified into five groups:
- Pulmonary arterial hypertension (PAH). This group consists of idiopathic PAH and PAH due to connective tissue diseases, HIV infection, portal hypertension, congenital heart disease, schistosomiasis, chronic hemolytic anemia, persistent pulmonary hypertension of the newborn, pulmonary veno-occlusive disease, drug- and toxin-induced PH and pulmonary capillary hemangiomatosis.
- Pulmonary hypertension owing to left heart disease. PH due to systolic dysfunction, diastolic dysfunction, or valvular heart disease is included in this group.
- Pulmonary hypertension owing to lung diseases or hypoxemia. This group includes PH due to chronic obstructive pulmonary disease, interstitial lung disease, other pulmonary diseases with a mixed restrictive and obstructive pattern, sleep-disordered breathing, alveolar hypoventilation disorders, and other causes of hypoxemia [4].
- Chronic thromboembolic pulmonary hypertension. This group includes patients with PH due to thromboembolic occlusion of the proximal or distal pulmonary vasculature.
- Pulmonary hypertension with unclear multifactorial mechanisms. These patients have PH caused by hematologic disorders (eg, myeloproliferative disorders), systemic disorders (eg, sarcoidosis), metabolic disorders (eg, glycogen storage disease), or miscellaneous causes
Pulmonary arterial hypertension in congenital heart disease
In approximately 6% of adults with congenital heart disease PAH develops. In fact congenital heart disease has emerged to be one of the commonest associated causes of PAH.
The occurrence of PAH in congenital heart disease is usually the result of systemic-to-pulmonary shunting, leading to a blood volume overload of the pulmonary vasculature. Left to right shunts are most common; VSD, ASD, patent ductus arteriosus or AVSD.
Pathophysiology
The pathogenesis of PH is complex and just beginning to be elucidated. In patients with congenital heart disease, left-to-right intracardiac shunting increases flow through the pulmonary vasculature, this causes shear forces that disrupt the vascular endothelium and activate cellular mechanisms critical to the pathogenesis and progression of PAH.
The mechanism of damage to the pulmonary vasculature differs in patients with ASD compared to VSD. Vascular injury is related to the degree and duration of volume overload alone with an ASD, whereas high pressure shear forces also contribute with a VSD.
In patients with an ASD, shunting is delayed until maturation of the pulmonary vasculature occurs. The normal pulmonary vasculature is able to accommodate the increased volume of flow by vasodilating and recruiting previously unperfused vessels; thus, pulmonary artery pressures do not rise significantly in most patients with an ASD until adult life. In contrast to patients with an ASD, clinical sequelae always develop in patients with a large (nonrestrictive) VSD. Severe PAH is present from birth because of the unique hemodynamics. The combined effect of volume overload and shear forces elevates the pulmonary vascular resistance, which becomes fixed during childhood. As a result, shunt reversal (right-to-left flow) is common, with hypoxemia resulting. This is often referred to as the Eisenmenger syndrome.
Eisenmenger syndrome forms a small percentage (1%) of CHD patients and is defined as CHD with an initial large systemic-to-pulmonary shunt that induces progressive pulmonary vascular disease and PAH, with resultant reversal of the shunt and central cyanosis. Eisenmenger syndrome represents the most advanced form of PAH associated with CHD. An important subgroup in this Eisenmenger population is patients with Down syndrome.
Classification
Pulmonary arterial hypertension in congenital heart disease can be divided into four quite distinct phenotypes, which may be feasible to use in clinical practice.
A. Eisenmenger syndrome. This includes all systemic-to-pulmonary shunts resulting from large defects and leading to a severe increase in pulmonary vascular resistance (PVR) and a reversed (pulmonary-to-systemic) or bidirectional shunt; cyanosis, erythrocytosis, and multiple organ involvement are present.
B. PAH associated with systemic-to-pulmonary shunts. This includes moderate to large defects; PVR is mildly to moderately increased, systemic-to-pulmonary shunt is still prevalent, and no cyanosis is present at rest.
C. PAH associated with small defects. This includes small defects (usually VSD<1 cm and ASD <2 cm of effective diameter assessed by echocardiography).
D. PAH after corrective cardiac surgery. In this group of patients the congenital heart disease has been corrected, but PAH is still present immediately after surgery or recurs several months or years after surgery in the absence of significant postoperative residual lesions.
Treatment
Early treatment of PH is generally suggested because advanced disease may be less responsive to therapy. PAH is characterized by symptoms of dyspnea, fatigue, chest pain, and syncope. Primary therapeutic strategy should be considered in all patients with PAH and consists of diuretic, oxygen, anticoagulant, and digoxin therapy, as well as exercise and lifestyle advices.
Injured endothelial cells release factors that are known to contribute to PAH. Inhibition of these factors forms the basis of some of the advanced therapies for PAH. Patients with CHD-PAH who progress to NYHA functional class II, III, or IV, qualify for advanced therapy. This is irrespective of any primary therapy given.
Advanced therapy is directed at the PAH itself, rather than the underlying cause of the PAH. For PAH treatments with advanced therapy three main pathways have been detected: prostacyclin, nitric oxide and endothelin-1.This resulted in therapies with prostanoids such as epoprostenol, phosphodiesterase-5 inhibitors such as sildenafil and endothelin-1 receptor antagonists such as bosentan.
Outcome
The prognosis is generally poor but varies according to the severity of the underlying cause, the functional abnormalities, and the hemodynamic abnormalities. Factors that may indicate a poor prognosis include age at presentation greater than 45 years, NYHA functional class III or IV, failure to improve to a lower NYHA functional class during treatment, pericardial effusion, large right atrial size, elevated right atrial pressure, septal shift during diastole, decreased pulmonary arterial capacitance (ie, the stroke volume divided by the pulmonary arterial pulse pressure) and increased N-terminal brain natriuretic peptide level.
Therapy improves exercise capacity and functional class, however the impact on mortality has been less well established.
References
-
Basow, D. S. (2012a). Classification and clinical features of isolated atrial septal defects in children. UpToDate. Waltham, MA.: UpToDate.
-
Basow, D. S. (2012b). Management of atrial septal defects in adults. UpToDate. Waltham, MA.: UpToDate.
-
Basow, D. S. (2012c). Management and outcome of isolated atrial septal defects in children. UpToDate. Waltham, MA.: UpToDate.
-
Basow, D. S. (2012d). Pathophysiology and clinical features of atrial septal defects in adults. UpToDate. Waltham, MA.: UpToDate.
-
Basow, D. S. (2012e). Identification and assessment of atrial septal defects in adults. UpToDate. Waltham, MA.: UpToDate.
-
Basow, D. S. (2012f). Devices for percutaneous closure of a secundum atrial septal defect. UpToDate. Waltham, MA.: UpToDate.
-
Berger, F., Vogel, M., Alexi-Meskishvili, V., & Lange, P. E. (1999). Comparison of results and complications of surgical and Amplatzer device closure of atrial septal defects. The Journal of Thoracic and Cardiovascular Surgery, 118(4), 674-678; discussion 678-680
-
Engelfriet, P., Boersma, E., Oechslin, E., Tijssen, J., Gatzoulis, M. A., Thilén, U., Kaemmerer, H., e.a. (2005). The spectrum of adult congenital heart disease in Europe: morbidity and mortality in a 5 year follow-up period. The Euro Heart Survey on adult congenital heart disease. European Heart Journal, 26(21), 2325-2333. doi:10.1093/eurheartj/ehi396
-
Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006a). Atrial Septal Defect. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum.
-
Roos-Hesselink, J. W., Meijboom, F. J., Spitaels, S. E. C., van Domburg, R., van Rijen, E. H. M., Utens, E. M. W. J., Bogers, A. J. J. C., e.a. (2003). Excellent survival and low incidence of arrhythmias, stroke and heart failure long-term after surgical ASD closure at young age. A prospective follow-up study of 21-33 years. European Heart Journal, 24(2), 190-197.
-
Basow, D. S. (2012h). Pathophysiology and clinical features of isolated ventricular septal defects in infants and children. UpToDate. Waltham, MA.: UpToDate.
-
Basow, D. S. (2012i). Management of isolated ventricular septal defects in infants and children. UpToDate. Waltham, MA.: UpToDate.
-
Baumgartner, H., Bonhoeffer, P., De Groot, N. M. S., de Haan, F., Deanfield, J. E., Galie, N., Gatzoulis, M. A., e.a. (2010). ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). European Heart Journal, 31(23), 2915-2957.
-
Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006b). Ventricular Septal Defect. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum.
-
Verheugt, C. L., Uiterwaal, C. S. P. M., Grobbee, D. E., & Mulder, B. J. M. (2008). Long-term prognosis of congenital heart defects: a systematic review. International Journal of Cardiology, 131(1), 25-32.
-
Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Patent Ductus Arteriosus. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum
-
Rudolph, A. M. (1970). The changes in the circulation after birth. Their importance in congenital heart disease. Circulation, 41(2), 343-359.
-
Ijland, M. M., & Tanke, R. B. (2009). Aortic coarctation. Circulation, 120(13), 1294-1295.
-
Luijendijk, P, Boekholdt, S. M., Blom, N. A., Groenink, M., Backx, A. P., Bouma, B. J., Mulder, B. J. M., e.a. (2011). Percutaneous treatment of native aortic coarctation in adults. Netherlands Heart Journal: Monthly Journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation, 19(10), 436-439.
-
Luijendijk, Paul, Bouma, B. J., Vriend, J. W. J., Vliegen, H. W., Groenink, M., & Mulder, B. J. M. (2011). Usefulness of exercise-induced hypertension as predictor of chronic hypertension in adults after operative therapy for aortic isthmic coarctation in childhood. The American Journal of Cardiology, 108(3), 435-439.
-
Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Coarctation of the aorta. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum.
-
Vriend, J. W. J., & Mulder, B. J. M. (2005). Late complications in patients after repair of aortic coarctation: implications for management. International Journal of Cardiology, 101(3), 399-406.
-
Vriend, J. W. J., Oosterhof, T., & Mulder, B. (2005). Noninvasive imaging for the postoperative assessment of aortic coarctation patients. Chest, 127(6), 2295.
-
Drenthen, W., Pieper, P. G., Ploeg, M., Voors, A. A., Roos-Hesselink, J. W., Mulder, B. J. M., Vliegen, H. W., e.a. (2005). Risk of complications during pregnancy after Senning or Mustard (atrial) repair of complete transposition of the great arteries. European Heart Journal, 26(23), 2588-2595. doi:10.1093/eurheartj/ehi472
-
Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Transposition of the great arteries. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum.
-
van der Zedde, J., Oosterhof, T., Tulevski, I. I., Vliegen, H. W., & Mulder, B. J. M. (2005). Comparison of segmental and global systemic ventricular function at rest and during dobutamine stress between patients with transposition and congenitally corrected transposition. Cardiology in the Young, 15(2), 148-153. doi:10.1017/S1047951105000326
-
Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Congenitally corrected transposition of the great arteries. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum.
-
Winlaw, D. S., McGuirk, S. P., Balmer, C., Langley, S. M., Griselli, M., Stümper, O., De Giovanni, J. V., e.a. (2005). Intention-to-treat analysis of pulmonary artery banding in conditions with a morphological right ventricle in the systemic circulation with a view to anatomic biventricular repair. Circulation, 111(4), 405-411.
-
Winter, M. M., Bouma, B. J., van Dijk, A. P. J., Groenink, M., Nieuwkerk, P. T., van der Plas, M. N., Sieswerda, G. T., e.a. (2008). Relation of physical activity, cardiac function, exercise capacity, and quality of life in patients with a systemic right ventricle. The American Journal of Cardiology, 102(9), 1258-1262.
-
Winter, M. M., van der Bom, T., de Vries, L. C. S., Balducci, A., Bouma, B. J., Pieper, P. G., van Dijk, A. P. J., e.a. (2011). Exercise training improves exercise capacity in adult patients with a systemic right ventricle: a randomized clinical trial. European Heart Journal.
-
Winter, M. M., van der Plas, M. N., Bouma, B. J., Groenink, M., Bresser, P., & Mulder, B. J. M. (2010). Mechanisms for cardiac output augmentation in patients with a systemic right ventricle. International Journal of Cardiology, 143(2), 141-146.
-
Basow, D. S. (2012). Hypoplastic left heart syndrome. UpToDate. Waltham, MA.: UpToDate.
-
Basow, D. S. (2012). Hypoplastic left heart syndrome. UpToDate. Waltham, MA.: UpToDate.
-
Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Univentricular heart and the Fontan circulation. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum.
-
Schuuring, M. J., Vis, J. C., Bouma, B. J., van Dijk, A. P. J., van Melle, J. P., Pieper, P. G., Vliegen, H. W., e.a. (2011). Rationale and design of a trial on the role of bosentan in Fontan patients: Improvement of exercise capacity? Contemporary Clinical Trials, 32(4), 586-591.
-
Schuuring, Mark J, Vis, J. C., Duffels, M. G., Bouma, B. J., & Mulder, B. J. (2010). Adult patients with pulmonary arterial hypertension due to congenital heart disease: a review on advanced medical treatment with bosentan. Therapeutics and Clinical Risk Management, 6, 359-366.
-
van den Bosch, A. E., Roos-Hesselink, J. W., Van Domburg, R., Bogers, A. J. J. C., Simoons, M. L., & Meijboom, F. J. (2004). Long-term outcome and quality of life in adult patients after the Fontan operation. The American Journal of Cardiology, 93(9), 1141-1145.
-
Balci, A., Drenthen, W., Mulder, B. J. M., Roos-Hesselink, J. W., Voors, A. A., Vliegen, H. W., Moons, P., e.a. (2011). Pregnancy in women with corrected tetralogy of Fallot: occurrence and predictors of adverse events. American Heart Journal, 161(2), 307-313.
-
Lillehei, C. W., Cohen, M., Warden, H. E., Read, R. C., Aust, J. B., Dewall, R. A., & Varco, R. L. (1955). Direct vision intracardiac surgical correction of the tetralogy of Fallot, pentalogy of Fallot, and pulmonary atresia defects; report of first ten cases. Annals of Surgery, 142(3), 418-442.
-
Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Tetralogy of Fallot. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum.
-
Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Marfan’s syndrome. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum.
-
Mulder, Barbara J M, & van der Wall, E. E. (2009). Tetralogy of Fallot: in good shape? The International Journal of Cardiovascular Imaging, 25(3), 271-275.
-
Oosterhof, T., Mulder, B. J. M., Vliegen, H. W., & de Roos, A. (2006). Cardiovascular magnetic resonance in the follow-up of patients with corrected tetralogy of Fallot: a review. American Heart Journal, 151(2), 265-272.
-
Vliegen, H. W., van Straten, A., de Roos, A., Roest, A. A. W., Schoof, P. H., Zwinderman, A. H., Ottenkamp, J., e.a. (2002). Magnetic resonance imaging to assess the hemodynamic effects of pulmonary valve replacement in adults late after repair of tetralogy of fallot. Circulation, 106(13), 1703-1707.
-
Windhausen, F., Boekholdt, S. M., Bouma, B. J., Groenink, M., Backx, A. P. C. M., de Winter, R. J., Mulder, B. J. M., e.a. (2011). Per-operative stent placement in the right pulmonary artery; a hybrid technique for the management of pulmonary artery branch stenosis at the time of pulmonary valve replacement in adult Fallot patients. Netherlands Heart Journal: Monthly Journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation, 19(10), 432-435.
-
de Witte, Piet, Aalberts, J. J. J., Radonic, T., Timmermans, J., Scholte, A. J., Zwinderman, A. H., Mulder, B. J. M., e.a. (2011). Intrinsic biventricular dysfunction in Marfan syndrome. Heart (British Cardiac Society), 97(24), 2063-2068.
-
Engelfriet, P., & Mulder, B. (2007). Is there benefit of beta-blocking agents in the treatment of patients with the Marfan syndrome? International Journal of Cardiology, 114(3), 300-302.
-
Radonic, T, de Witte, P., Groenink, M., de Bruin-Bon, R., Timmermans, J., Scholte, A., van den Berg, M., e.a. (2011). Critical appraisal of the revised Ghent criteria for diagnosis of Marfan syndrome. Clinical Genetics.
-
Basow, D. S. (2012). Ebstein’s anomaly of the tricuspid valve. UpToDate. Waltham, MA.: UpToDate.
-
Carpentier, A., Chauvaud, S., Macé, L., Relland, J., Mihaileanu, S., Marino, J. P., Abry, B., e.a. (1988). A new reconstructive operation for Ebstein’s anomaly of the tricuspid valve. The Journal of Thoracic and Cardiovascular Surgery, 96(1), 92-101.
-
Celermajer, D. S., Bull, C., Till, J. A., Cullen, S., Vassillikos, V. P., Sullivan, I. D., Allan, L., e.a. (1994). Ebstein’s anomaly: presentation and outcome from fetus to adult. Journal of the American College of Cardiology, 23(1), 170-176.
-
Ebstein W. (1866) Ueber einen sehr seltenen Fall von Insufficienz der Valvula tricuspidalis, bedingt durch eine angeborene hochgradige Missbildung. Arch Anat physiol;33:238.
-
Mulder, B. J. M. (2002). Ebstein’s anomaly in adults. The International Journal of Cardiovascular Imaging, 18(6), 439-441.
-
Mulder, B. J. M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Ebstein anomaly of the tricuspid valve. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum.
-
Beghetti, Maurice, & Tissot, C. (2009). Pulmonary arterial hypertension in congenital heart diseases. Seminars in Respiratory and Critical Care Medicine, 30(4), 421-428.
-
Beghetti, Maurice, & Tissot, C. (2010). Pulmonary hypertension in congenital shunts. Revista Española De Cardiología, 63(10), 1179-1193.
-
Duffels, M G J, Engelfriet, P. M., Berger, R. M. F., van Loon, R. L. E., Hoendermis, E., Vriend, J. W. J., van der Velde, E. T., e.a. (2007). Pulmonary arterial hypertension in congenital heart disease: an epidemiologic perspective from a Dutch registry. International Journal of Cardiology, 120(2), 198-204.
-
Engelfriet, Peter M, Duffels, M. G. J., Möller, T., Boersma, E., Tijssen, J. G. P., Thaulow, E., Gatzoulis, M. A., e.a. (2007). Pulmonary arterial hypertension in adults born with a heart septal defect: the Euro Heart Survey on adult congenital heart disease. Heart (British Cardiac Society), 93(6), 682-687.
-
Galie, N., Hoeper, M. M., Humbert, M., Torbicki, A., Vachiery, J.-L., Barbera, J. A., Beghetti, M., e.a. (2009). Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). European Heart Journal, 30, 2493-2537.
-
Gatzoulis, M A, Alonso-Gonzalez, R., & Beghetti, M. (2009). Pulmonary arterial hypertension in paediatric and adult patients with congenital heart disease. European Respiratory Review: An Official Journal of the European Respiratory Society, 18(113), 154-161.
-
Lau, E. M. T., Manes, A., Celermajer, D. S., & Galiè, N. (2011). Early detection of pulmonary vascular disease in pulmonary arterial hypertension: time to move forward. European Heart Journal, 32(20), 2489-2498.
-
Mulder, B J M. (2010). Changing demographics of pulmonary arterial hypertension in congenital heart disease. European Respiratory Review: An Official Journal of the European Respiratory Society, 19(118), 308-313. doi:10.1183/09059180.00007910
-
Mulder, B.J.M., Pieper, P. G., Meijboom, F. J., & Hamer, J. P. M. (2006). Eisenmenger syndrome. Adult Congenital Heart Disease (Aangeboren hartafwijkingen bij volwassenen) (Second edition.). Houten: Bohn Stafleu van Loghum.
-
Schuuring, M J, van Riel, A. C. M. J., Bouma, B. J., & Mulder, B. J. M. (2011). Recent progress in treatment of pulmonary arterial hypertension due to congenital heart disease. Netherlands Heart Journal: Monthly Journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation, 19(12), 495-497.
-
Simonneau, G., Robbins, I. M., Beghetti, M., Channick, R. N., Delcroix, M., Denton, C. P., Elliott, C. G., e.a. (2009). Updated clinical classification of pulmonary hypertension. Journal of the American College of Cardiology, 54(1 Suppl), S43-54.
-
Vis, J. C., Duffels, M. G., Mulder, P., de Bruin-Bon, R. H. A. C. M., Bouma, B. J., Berger, R. M. F., Hoendermis, E. S., e.a. (2011). Prolonged beneficial effect of bosentan treatment and 4-year survival rates in adult patients with pulmonary arterial hypertension associated with congenital heart disease. International Journal of Cardiology.