Cardiac Pharmacology: Difference between revisions
No edit summary |
|||
| (3 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
''Heather Melrose, Jonas de Jong'' | ''Heather Melrose, Jonas de Jong'' | ||
| Line 7: | Line 6: | ||
==Renin-Angiotensin-Aldosterone System== | ==Renin-Angiotensin-Aldosterone System== | ||
[[Image:Renin-angiotensin-aldosterone_system.png|thumb|right|500px|RAAS schematic]] | |||
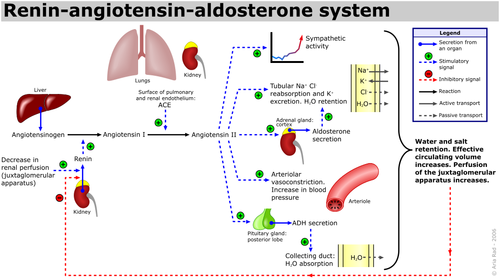
The renin-angiotensin-aldosterone system (RAAS) is an important hormone-based pathway within the body that regulates fluid balance and thus systemic blood pressure. The system is activated by decreases in blood volume or pressure detected in two ways: a drop in blood pressure detected by baroreceptors (pressure sensors) located in the carotid sinus or a drop in flow rate through the kidneys, detected by the juxtaglomerular apparatus. The body responds to these stimuli to effect a restoration in blood pressure via the actions of three hormones; renin, angiotensin and aldosterone. Following the detected drop in blood pressure, the enzyme renin is released from specialised cells within the kidney. The substrate of renin is the inactive precursor of angiotensin I, angiotensinogen. Angiotensin I is then enzymatically converted by angiotensin converting enzyme (ACE) into angiotensin II, a hormone with various actions throughout the body that ultimately increase blood pressure, restoring fluid balance within the body. | The renin-angiotensin-aldosterone system (RAAS) is an important hormone-based pathway within the body that regulates fluid balance and thus systemic blood pressure. The system is activated by decreases in blood volume or pressure detected in two ways: a drop in blood pressure detected by baroreceptors (pressure sensors) located in the carotid sinus or a drop in flow rate through the kidneys, detected by the juxtaglomerular apparatus. The body responds to these stimuli to effect a restoration in blood pressure via the actions of three hormones; renin, angiotensin and aldosterone. Following the detected drop in blood pressure, the enzyme renin is released from specialised cells within the kidney. The substrate of renin is the inactive precursor of angiotensin I, angiotensinogen. Angiotensin I is then enzymatically converted by angiotensin converting enzyme (ACE) into angiotensin II, a hormone with various actions throughout the body that ultimately increase blood pressure, restoring fluid balance within the body. | ||
Revision as of 08:41, 28 May 2015
Heather Melrose, Jonas de Jong
Cardiovascular disease including heart disease, arrhythmias and hypertension, is the leading cause of morbidity and mortality in the Western world. There are numerous devastating conditions affecting the heart and/or the vasculature, leading to high demand for cardiovascular drugs. This chapter focuses on some key therapeutic targets within the cardiovascular system and the drugs used to combat cardiovascular disease.
Renin-Angiotensin-Aldosterone System
The renin-angiotensin-aldosterone system (RAAS) is an important hormone-based pathway within the body that regulates fluid balance and thus systemic blood pressure. The system is activated by decreases in blood volume or pressure detected in two ways: a drop in blood pressure detected by baroreceptors (pressure sensors) located in the carotid sinus or a drop in flow rate through the kidneys, detected by the juxtaglomerular apparatus. The body responds to these stimuli to effect a restoration in blood pressure via the actions of three hormones; renin, angiotensin and aldosterone. Following the detected drop in blood pressure, the enzyme renin is released from specialised cells within the kidney. The substrate of renin is the inactive precursor of angiotensin I, angiotensinogen. Angiotensin I is then enzymatically converted by angiotensin converting enzyme (ACE) into angiotensin II, a hormone with various actions throughout the body that ultimately increase blood pressure, restoring fluid balance within the body.
Angiotensin II causes increases in blood pressure by actions at various sites:
- Adrenal Glands: Angiotensin II augments release of the steroid hormone aldosterone, which acts locally to augment sodium retention and potassium secretion from the kidney. The net effect of this is water retention, thus restoring fluid balance.
- Kidneys: Angiotensin II also increases sodium retention via direct actions on renal proximal tubules, as well as affecting glomerular filtration rate and renal blood flow.
- Cardiovascular System: Angiotensin II is a potent endogenous vasoconstrictor, causing resistance arteries and veins to constrict, raising blood pressure. Furthermore in both the blood vessels and the heart, prolonged increases in Angiotensin II encourage cell growth and resultant hypertrophy.
- Central Nervous System: In the brain, Angiotensin II acts on the posterior pituitary gland, stimulating release of antidiuretic hormone (ADH, also known as Arginine Vasopressin (AVP)). ADH increases water reabsorption in the renal collecting ducts. Angiotensin II also acts on the subfornical organ within the brain to cause increased thirst, encouraging water intake.
Chronic activation of the RAAS system can lead to deleterious remodelling and increased inflammation in the heart, vasculature and kidneys, as well as hypertension and chronic kidney disease.
Neural Control of the Cardiovascular System
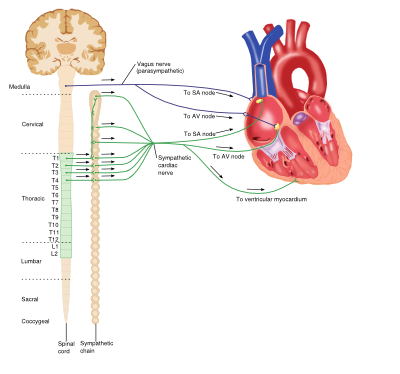
Sympathetic (Adrenergic) Nervous System
The adrenergic nervous system is a vital component of many processes throughout the body, including the cardiovascular system. Circulating catecholamines (e.g. adrenaline and noradrenaline) bind to and activate adrenergic receptors on cell membranes. Adrenergic receptors are a class of G-protein coupled receptors that elicit a variety of tissue-specific effects and exist in several subtypes.
Vasculature
The predominant receptor subtype present in blood vessels is the a1-adrenergic receptor, activation of which by catecholamine binding causes activation of the phospholipase-C (PLC), inositol triphosphate (IP3), diacylglycerol (DAG) intracellular signalling pathway. This ultimately results in myocyte contraction, vasoconstriction and consequent increases in systemic blood pressure.
Heart
Although the heart is myogenic, that is the impetus for contraction is self-initiated, the output of the heart is influenced by the central nervous system. The net effect of the sympathetic system on the heart is to increase cardiac output. The adrenergic receptors found in the heart belong to the ß-receptor subfamily and include ß1 and ß3 receptors. Catecholamine binding to ß1-receptors in the heart causes increases in cardiac output via a number of mechanisms: positive chronotropic effects, positive inotropic effects increased automaticity and conduction in both ventricular myocytes and the atrioventricular (AV) node. However ß3-receptor activation antagonises these actions, producing a negative inotropic effect and providing an inbuilt control system within the heart.
Prolonged increase catecholamine levels in the circulation (e.g. when secreted from adrenal tumours or times of stress) can lead to chronic cardiovascular problems such as hypertension and arrhythmias.
Parasympathetic Nervous System
The parasympathetic system relies on the binding of the neurotransmitter acetylcholine (Ach) to muscarinic receptors, and has various roles throughout the body.
Vasculature
Although blood vessels do express muscarinic receptors, they do not receive cholinergic innervation; however application of exogenous Ach results in a swift and profound vasodilation.
Heart
Activation of muscarinic receptors (M2-subtype) in the heart by Ach released from the vagus nerve causes a reduction in cardiac output via opposite effects to adrenergic stimulation: negative chronotropic effects and decreases in AV node conduction as well as decreasing the force of atrial contractions.
Platelet/Clotting System
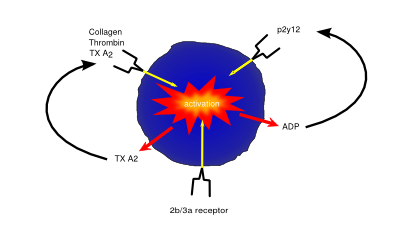
Platelets (also known as thrombocytes) are small cells lacking nuclei that are responsible for haemostasis, or blood clotting. Damage or injury leading to blood loss and exposure of extracellular collagen fibres is detected, activating platelets. Once activated, platelets become adhesive, sticking to both the damaged vessel wall and each other, forming a clump of cells, or ‘clot’, helping to dam the vessel leak. They then begin to secrete cytokines that encourage invasion of fibroblasts present in the surrounding tissue which form a more permanent patch, either by creating healthy tissue, or depositing extracellular matrix to form a scar.
There are several conditions in which abnormal clotting can be damaging to the body; excess clotting can lead to vascular blockage and ischaemia or stroke; less commonly, deficient clotting can lead to excess blood loss, for example in haemophilia. To combat these diseases, there are drugs that modulate the clotting process.
Anti-coagulants
Drugs that prevent clotting (anti-coagulants) are important in those with an increased risk of clotting-mediated damage such as a stroke or ischaemia.
As well being an analgesic and anti-pyretic, Aspirin is an anti-thrombotic agent given in low doses to those at risk of damage from clotting (e.g. following a heart attack). Aspirin’s anti-coagulant actions come from its suppression of key pro-clotting factors such as prostaglanding and thromboxanes via irreversible inactivation of the PTGS cyclooxygenase enzyme. This suppression of factors such as thromboxane A2 reduces platelet aggregation and thus prevents clot formation.
P2Y12 inhibitors such as clopidogrel exert their anti-coagulant effect via inhibition of the P2Y12 subtype of the platelet ADP receptor. By blocking P2Y12, these drugs prevent activation of platelets and the formation of the fibrin network needed for clotting.
Drugs such as abciximab and tirofiban prevent clotting via inhibition of the glycoprotein IIb/IIIa receptor preventing both platelet activation and aggregation.
Pharmacokinetics
When administering drugs to a patient, it is crucial to know several facts about the drug in order to maximise efficacy and minimise side-effects/toxicity. These include information about what dose is effective, how long the drug remains active in the body, how quickly it is broken down/removed from the body, and how easily the body can absorb/use that drug. The following table details these pharmacokinetic properties and how they are calculated:
| Property | Description | Standard units (Abbreviation) | Formula |
|---|---|---|---|
| Dose | Amount of active drug given to patient | mg (D) | Drug Specific (From clinical studies) |
| Concentration | Amount of drug in a given plasma volume | µg/ml (C) | = D / Vd |
| EC50 | The concentration of drug needed to elicit a response halfway between zero and maximal responses. | µg/ml (EC50) | y = bottom + (Top-Bottom)/(1+ [x/EC50] Hill Coefficient) |
| Volume of Distribution | The theoretical volume the drug would occupy if distributed uniformly throughout the tissues to elicit the current plasma concentration. | L (Vd) | D / C |
| Elimination Constant (Rate) | The rate at which the drug is removed from the body. | h-1 (Ke) | ln(2) / t1/2 or CL / Vd |
| Bioavailability | How much of the administered dose is available for actual use by the body. | no units as expressing a fraction (f) | 100 × (AUC (po)×D (iv))/(AUC (iv)×D (po))
AUC = Area under curve po = oral administration iv = intravenous administration |
| Cmax or Cmin | The maximum (Cmax) / minimum (Cmin) plasma drug concentration reached following drug administration | µg/ml (Cmax or Cmin) | Identified via direct measurement of plasma C |
| tmax | The time it takes for a drug to reach Cmax following administration | h (tmax) | Identified via direct measurement of plasma C over time |
| Half-life | The time it takes for a drug to reach half its original concentration | h (t1/2) | ln(2) / Ke |
| Drug Clearance | The volume of plasma cleared of the drug over a set time | l/h (CL) | Vd x Ke or D / Area under curve |
Common Drug-Drug Interactions
It is important to be aware of the interactions that can occur between concomitantly administered drugs, as they may effect efficacy and/or toxicity, or produce adverse side effects. Such interactions could for example affect drug absorption, drug bioavailability or efficacy, or combine to produce unwanted metabolites, as well as possibly having effects on clinical analyses. If a combination of two drugs decreases the effect of one or both of them, the interaction is termed an antagonistic effect; however if, conversely, a combination of two drugs enhances the effect of one or both of them, the interaction is termed a synergistic effect. Drugs that act on the cardiovascular system are high in interactivity, which is an issue as cardiovascular patients normally receive more than one drug. Some common drug—drug interactions related to cardiovascular drugs are listed below:
| Drug | Drugs that ↑ drug action | Drugs that ↓ drug action |
|---|---|---|
| Digoxin |
|
|
| Warfarin |
|
|
| Clopidogrel |
|
|
| Furosemide |
| |
| ACE Inhibitors |
|
|
| ß-blockers |
|
|
| Statins |
|
|
There are several mechanisms by which drugs are broken down by the body, usually via degradation by enzymes. One common family of enzymes involved in drug metabolismis the cytochrome P450 (CYP) family; a large, diverse group of enzymes that encourage oxidation of a variety of substrates, both endogenous (e.g. steroid hormones) and exogenous (e.g. toxins and drugs). CYP enzymes account for up to 75% of drug metabolism, aiding some drugs to form their active compounds but mostly deactivating drugs into inactive metabolites to be excreted. CYP enzymes can influence drug actions in several ways; they can increase drug metabolism (either increasing action via formation of the active by-product or decreasing action by metabolism of the active drug) or their action can be inhibited by drugs that compete for access to the CYP enzymes active site, preventing the normal interaction between drug and enzyme. Many drugs exert their interactions with other drugs viainterference with the CYP system. For example, if Drug A is metabolised by CYP and Drug B inhibits CYP activity, co-administration will result in a decreased bioavailability of Drug A. In humans there are 18 families and 43 subfamilies of the CYP group of enzymes, which target different substrates. Some CYP enzymes important in cardiovascular medicine, their cardiovascular-drug substrates and some of their interactions are shown in the table below:
| Enzyme | Substrates (e.g.) | Inhibitors (e.g.) | Inducers (e.g.) |
|---|---|---|---|
| CYP2C19 |
|
|
|
| CYP3A4 |
|
|
|
| CYP2C9 |
|
|
|
| CYP2D6 |
|
|
|
In addition to drug-drug interactions, the actions of many drugs are also affected by food or drink. For example, care should be taken with alcohol consumption with many kinds of drugs, as it can put stress on the liver which is already working hard to metabolise drugs in the body. Grapefruit juice too can cause issues, as it is known to inhibit CYP3a. For more information of interactions between drugs and food/drinks see this guide: General Use of Medicine
| Drug Type | Examples (generic name) | Indications | Typical Dosage | Guidelines / Class of Indication | Side Effects (Prevalence %) |
|---|---|---|---|---|---|
| :Anti-hypertensives | |||||
| Diuretics | Furosemide | Oedema | Furosemide: 20-40mg once daily | Mild gastro-intestinal disturbances, pancreatitis, hepatic encephalopathy, postural hypotension, temporary increase in serum-cholesterol and triglyceride concentration, hyperglycaemia, acute urinary retention, electrolyte disturbances, metabolic alkalosis, blood disorders, hyperuricaemia, visual disturbances, tinnitus and deafness, and hypersensitivity reactions (including rash, photosensitivity, and pruritus). | |
| Resistant Hypertension | Furosemide: 40-80mg once daily | Hypertension in symptomatic (NYHA class II-IV) HF and LVD: Class IC [1] | |||
| ACE Inhibitors | Captopril, Monopril | Hypertension | Captopril: 12.5mg twice daily | Hypertension: Class IA [2]
Hypertension in symptomatic (NYHA class II-IV) HF and LVD: Class IA [1] Hypertension in diabetics: Class IA [3] |
Hypotension (2.4%), renal impairment, persistent dry cough, angioedema, rash pancreatitis, upper respiratory-tract symptoms (2-10%), gastro-intestinal symptoms (1-2%), altered liver function tests, cholestatic jaundice, hepatitis, fulminant hepatic necrosis and failure, hyperkalaemia (2%), hypoglycaemia, blood disorders including thrombocytopenia, leucopenia, neutropenia, headache (3%), dizziness (2-12%), fatigue, malaise, taste disturbance, paraesthesia, bronchospasm, fever, serositis, vasculitis, myalgia (3%), arthralgia, positive antinuclear antibody, raised erythrocyte sedimentation rate, eosinophilia, leucocytosis, and photosensitivity. |
| Heart Failure | Captopril: 12.5mg 3 times daily | Post STEMI: Class IA [4]
Diabetic patients: Class IC [2] Symptomatic (NYHA class II-IV) HF: Class IA; Acute heart failure with ACS: Class IA [1] | |||
| Prophylaxis Following MI | Captopril: 6.25mg once daily | ||||
| Diabetic nephropathy | Captopril: 75-100mg once daily | ||||
| Angiotensin Receptor Blockers | Losartan, Candesartan | Hypertension | Losartan: 50mg once daily | Hypertension: Class IA [2]
Hypertension in diabetics: Class IA [3] |
Gastro-intestinal disturbances (<3%), dizziness (14%), angina, palpitation, oedema, dyspnoea, headache (14%), malaise, urticaria, pruritus, rash; |
| Left ventricular hypertrophy | Losartan: 12.5-150mg daily | LVH: Class IB [4] | |||
| Diabetic nephropathy | Losartan: 50mg daily | ||||
| Alpha Blockers | Prazosin, Doxazosin | Hypertension | Prazosin: 1-10mg 2-3 times daily | Drowsiness, hypotension (notably postural hypotension) (10-70% initially), syncope (1%), asthenia, dizziness, depression, headache (8-18%), dry mouth, gastro-intestinal disturbances, oedema, blurred vision (<5%), intra-operative floppy iris syndrome, rhinitis (<4%), erectile disorders (including priapism), tachycardia and palpitations (7-14%), gastrointestinal side-symptoms (4-5%), hypersensitivity reactions including rash, pruritus and angioedema. | |
| Congestive Heart Failure | Prazosin: 4-20mg daily | ||||
| Raynaud’s Syndrome | Prazosin: 1-2mg daily | ||||
| Beta Blockers | Atenolol, Propranolol | Hypertension | Atenolol: 25-50mg daily | Gastro-intestinal disturbances (2-4%); bradycardia, heart failure, hypotension, conduction disorders, peripheral vasoconstriction, bronchospasm, dyspnoea; headache, fatigue, sleep disturbances (2-5%), paraesthesia, dizziness (2-5%), vertigo, psychoses; sexual dysfunction; purpura, thrombocytopenia; visual disturbances; exacerbation of psoriasis, alopecia; rarely rashes and dry eyes. | |
| Angina | Atenolol: 100mg once/twice daily | ACS: Class IIaB [2]
Angina in symptomatic (NYHA class II-IV) HF and LVD: Class IA [1] | |||
| Arrhythmias | Atenolol: 50-100mg daily | Atrial fibrillation: Class IA; Polymorphic VT: Class IB [4]
Symptomatic (NYHA class II-IV) HF, LVD and AF: Class IA; Management of VA in HF: Class IA [1] SVT: Class IIbC; Wide QRS-complex tachycardia of unknown origin: Class IIIC; Sinus tachycardia: Class IC; Poorly tolerated AVNRT with haemodynamic intolerance: Class IIaC; Recurrent symptomatic AVNRT: Class IC; Documented PSVT with only dual AV-nodal pathways or single echo beats demonstrated during electrophysiological study and no other identified cause of arrhythmia: Class IC; Infrequent, well tolerated AVNRT: Class IB; Focal junction tachycardia: Class IIaC; Nonparoxysmal junctional tachycardia: Class IIaC; WPW Syndrome: Class IIaC; AVRT, poorly tolerated: Class IIbC; Since or infrequent AVRT episode(s): Class IIaB; Acute treatment of Focal Atrial Tachycardia: Class IIaC; Prophylactic therapy for AT: Class IC; AF (Poorly tolerated): Class IIaC; AF (Stable flutter): Class IC; Prophylaxis of SVT during pregnancy: Class IIaB [5] | |||
| Migraine | Atenolol: 50-200mg daily | ||||
| Calcium Channel Blockers | Nifedipine, Verapamil, Diltiazem | Hypertension | Nifedipine: 20-30mg once daily | Hypertension in symptomatic (NYHA class II-IV) HF and LVD: Class IA [1] | Gastro-intestinal disturbance (2-11%); hypotension (1-5%), oedema (7-29%), vasodilatation, palpitation; headache (7-35%), dizziness (3-27%), lethargy (4-6%), asthenia (10-12%); less commonly tachycardia (<1-7%), syncope (<1%), chills, nasal congestion, dyspnoea (<3%), anxiety, sleep disturbance (<2%), vertigo (<3%), migraine, paraesthesia, tremor (1-8%), polyuria, dysuria, nocturia, erectile dysfunction (<2%), epistaxis, myalgia, joint swelling, visual disturbance (<2%), sweating (<2%), hypersensitivity reactions (<1%); rarely anorexia, gum hyperplasia, mood disturbances, hyperglycaemia, male infertility, purpura (<1%), and photosensitivity reactions (<1%); also reported dysphagia, intestinal obstruction, intestinal ulcer, bezoar formation, gynaecomastia, agranulocytosis, and anaphylaxis; |
| Raynaud’s Syndrome | Nifedipine: 5-20mg 3 times daily | ||||
| Angina (prophylaxis) | Nifedipine: 5-20mg 3 times daily | Angina in symptomatic (NYHA class II-IV) HF and LVD: Class IIaA [1] | |||
| Anti-Arrhythmics | |||||
| Class I (sodium channel blockers) | Flecainide, Lidocaine, Procainamide | Ventricular Arrhythmias | Flecainide: 50-100mg twice daily | Sustained VT and VF: Class IIbC [4]
Pre-excited SVT/AF: Class IB; Wide QRS-complex tachycardia of unknown origin: Lidocaine (Class IIbB) / Procainamide (Class IB); Wide QRS-complex tachycardia of unknown origin with LVD: Class IB; Focal junction tachycardia: Class IIaC; WPW Syndrome: IIaC; AVRT, poorly tolerated: IIaC; Single or infrequent AVRT episode(s): Class IIbC; Acute treatment of Focal Atrial Tachycardia: Class IIaC; Prophylactic therapy for AT: Class IIaC; AF (Stable flutter): Class IIbA; Prophylaxis of SVT during pregnancy: Class IIbB [5] |
Oedema, pro-arrhythmic effects (1-13%); dyspnoea; nervous-system side-effects including dizziness, asthenia, fatigue, fever; visual disturbances (13-28%); rarely pneumonitis, hallucinations, depression, confusion, amnesia, dyskinesia, convulsions, peripheral neuropathy; also reported gastro-intestinal disturbances (1-4%), anorexia, hepatic dysfunction, flushing, syncope, drowsiness, tremor, vertigo, headache, anxiety, insomnia, ataxia, paraesthesia, anaemia, leucopenia, thrombocytopenia, corneal deposits, tinnitus, increased antinuclear antibodies, hypersensitivity reactions (including rash, urticaria, and photosensitivity), increased sweating. |
| Class II (Beta blockers) | (See above) | (See above) | (See above) | (See above) | |
| Class III (Potassium channel blockers) | Amiodarone, Sotalol | Ventricular, Arrhythmias | Amiodarone: 200mg 2-3 times daily | Sustained VT and VF: Class IIaC; Polymorphic VT: Class IC [4]
Management of VA in HF: Class IA; Prevention of VA in HF: Class IIbB [1] SVT: Class IIBC; Wide QRS-complex tachycardia of unknown origin: Class IB; Wide QRS-complex tachycardia of unknown origin with LVD: Class IB; Recurrent AVNRT unresponsive to beta blocker or calcium-channel blocker and patient not desiring RF ablation: Class IIbC; Focal junction tachycardia: Class IIaC; WPW Syndrome: IIaC; AVRT, poorly tolerated: Class IIaC; Since or infrequent AVRT episode(s): Class IIbB; Acute treatment of Focal Atrial Tachycardia: Class IIaC; Prophylactic therapy for AT: Class IIaC; AF (Poorly tolerated): Class IIbC; AF (Stable flutter): Class IIbC; Prophylaxis of SVT during pregnancy: Class IIIC [5] |
Gastro-intestinal disturbances (2-20%)), taste disturbances, hepatic disturbances (up to 50%); bradycardia; pulmonary toxicity (1-17%); tremor (9-59%), sleep disorders; hypothyroidism (5-10%), hyperthyroidism (5-10%); reversible corneal microdeposits (up to 98%); phototoxicity, persistent slate-grey skin discoloration (1-7%), injection-site reactions; less commonly onset or worsening of arrhythmia, conduction disturbances, peripheral neuropathy (1-105) and myopathy; very rarely sinus arrest, bronchospasm, ataxia (2-37%), benign intracranial hypertension, headache, vertigo, epididymo-orchitis, impotence, haemolytic or aplastic anaemia, thrombocytopenia, rash, hypersensitivity including photosensitivity (2-20%), anaphylaxis on rapid injection, hypotension (10-30%), respiratory distress syndrome, sweating, and hot flushes |
| Class IV (Calcium channel blockers) | (See above) | (See above) | (See above) | (See above) | |
| Digoxin | Supra-ventricular Arrhythmias | Acute: 0.75-1.5mg over 24 hours; Maintenance: 125-150µg daily | SVT: Class IIbC; WPW Syndrome: Class IIIC; AVRT, poorly tolerated: Class IIIC; Since or infrequent AVRT episode(s): Class IIIC; Prophylaxis of SVT during pregnancy: Class IC [5] | Gastro-intestinal disturbances (vomiting, diarrhoea, anorexia, abdominal pain) (25%); arrhythmias (up to 50%), AV conduction disturbances (50%); nervous system disturbances (dizziness, apathy, confusion, headache, fatigue, weakness) (25%); blurred or yellow vision; rash, eosinophilia, depression, anorexia, intestinal ischaemia and necrosis, psychosis, gynaecomastia on long-term use, and thrombocytopenia. | |
| Heart Failure | 62.5-125 µg daily | Symptomatic (NYHA class II-IV) HF: Class IIbB
Symptomatic (NYHA class II-IV) HF, LVD and AF: Class IB; Acute HF with AF and VT: Class IC [1] | |||
| Anti-platelet Drugs | |||||
| Aspirin | Prevention of thrombotic cerebro- or cardio-vascular disease | 75mg once/day | Prevention in AF: Class IC; Prevention in diabetic patients: IIaB [2]
Prevention in Symptomatic (NYHA class II-IV) HF and AF: Class IIA [1] Prevention in hypertensive patients with CV events: Class IA; Prevention in hypertensive patients without CV history but with reduced renal function/high risk: Class IIbA [3] Post-MI: Class Ia [3] |
Bronchospasm (10-30% in asthmatics); gastro-intestinal irritation (up to 83%), gastro-intestinal haemorrhage (occasionally major), also other haemorrhage (e.g. intracranial (0.5%), subconjunctival), chest pain (8.3%), oedema (4.5%), hypertension (4.3%). | |
| Pain / pyrexia | 300-600mg every 4-6 hours as necessary | ||||
| Clopidogrel | Prevention of thrombotic events (esp. when warfarin not tolerated) | 75mg once/day | Prevention in diabetic patients: IIaB; Primary and secondary prevention of stroke: Class IB [2]
Prevention in Symptomatic (NYHA class II-IV) HF and AF: Class IIA [1] Acute phase of coronary artery syndrome: Class IB; Non-cardioembolic cerebral ischaemic events: Class IA [3] |
Dyspepsia (5.2%), abdominal pain (5.6%), diarrhoea (4.5%); bleeding disorders including gastro-intestinal (2.0%) and intracranial (0.4%), nausea (3.4%), vomiting, gastritis, flatulence, constipation, gastric and duodenal ulcers, headache (7.6%), epistaxis (2.9%), dizziness (6.2%), paraesthesia, leucopenia, decreased platelets (very rarely severe thrombocytopenia), eosinophilia, rash (4.2%), pruritus (3.3%), vertigo, colitis, pancreatitis, hepatitis (<1%), acute liver failure, hypertension (4.3%), chest pain (8.3%), oedema (4.1%), vasculitis, confusion, hallucinations, taste disturbance, cough (3.9%), fatigue (4.8%) stomatitis, bronchospasm, interstitial pneumonitis, pyrexia (2.2%), blood disorders including thrombocytopenic purpura (5.3%), agranulocytosis, neutropenia (0.04%) and pancytopenia and hypersensitivity-like reactions (<0.1%)including fever, glomerulonephritis, arthralgia, Stevens-Johnson syndrome, toxic epidermal necrolysis, lichen planus. | |
| Acute myocardial infarction | 300mg daily initially then 75mg once/day | Post STEMI: Class IA [4] | |||
| Acute coronary syndrome | 300mg daily initially then 75mg once/day | ACS: Class IIaC [2] | |||
| Prasugrel | Prevention of thrombotic events. | 60mg bolus then 5-10mg once daily | Prevention in Symptomatic (NYHA class II-IV) HF and AF: Class IIA [1]
Acute phase of coronary artery syndrome: Class IB [3] |
Haemorrhage (11.3%) (including gastro-intestinal (1.5%) and intracranial), haematoma, haematuria, hypertension (7.5%), hypotension (3.9%), headache (5.5%), back pain (5.0%), dyspnoea (4.9%), nausea (4.6%), dizziness (4.1%), cough (3.9%), fatigue (3.7%), chest pain (3.1%), arrhythmias including atrial fibrillation (2.9%) and bradycardia (2.9%), rash (2.8%), pyrexia (2.7%), oedema (2.7%), diarrhoea (2.3%), hypercholesterolaemia/hyperlipidaemia (7.5%), anaemia, rash,hypersensitivity reactions including angioedema (0.06%), thrombocytopenia (0.06%), thrombotic thrombocytopenic purpura. | |
| Ticragelor | Prevention of thrombotic events. | 180mg bolus then 90mg twice daily | Prevention in Symptomatic (NYHA class II-IV) HF and AF: Class IIA [1]
Acute phase of coronary artery syndrome: Class IB [3] |
Dyspnoea (13.8%), haemorrhage, bruising; nausea (4.3%), vomiting, diarrhoea (3.7%), hypertension (3.8%), hypotension (3.2%), back pain (3.6%), abdominal pain, dyspepsia, gastritis, dizziness (4.5%), chest pain (3.7%), headache (6.5%), cough (4.9%), rash, pruritus, fatigue (3.2%), constipation, arrhythmias including atrial fibrillation (4.2%), paraesthesia, confusion, hyperuricaemia, raised serum creatinine (7.4%), vertigo. | |
| Vitamin K Antagonists | |||||
| Warfarin | Prevention of thrombotic/ embolic events (esp. after prosthetic valve insertion) | 5-10mg initially then tailored to individual (usually 3-9mg once daily at the same time) | Haemorrhage, nausea, vomiting, diarrhoea, jaundice, hepatic dysfunction, pancreatitis, pyrexia, alopecia, purpura, rash, ‘purple toes’, skin necrosis (increased risk in patients with protein C or protein S deficiency) | ||
| Acenocoumarol | Prevention of thrombotic/ embolic events (esp. after prosthetic valve insertion) | 4mg initially, followed by 1-8mg daily | Haemorrhage, nausea, vomiting, diarrhoea, jaundice, hepatic dysfunction, pancreatitis, pyrexia, alopecia, purpura, rash, ‘purple toes’, skin necrosis (increased risk in patients with protein C or protein S deficiency) | ||
| Lipid-Lowering Drugs | |||||
| Statins | Simvastatin, Atorvastatin | Primary hyper-cholesterolaemia, combined hyperlipidaemia | Simvastatin: 10-20mg once daily | Dyslipidaemia: Class IA; Low HDL-C: Class IIbB; Elderly patients with CVD: IB; Elderly patients with no CVD but CV risk factors: IIbB; Type I diabetes: IC; Patients with CKD: IIaC; Transplant patients: Class IIaB; PAD: Class IA; HIV patients: IIaC [2]
Hypertension in diabetics: Class IA; ACS: Class IA [3] |
Oedema (2.7%), abdominal pain (5.9%), nausea (5.4%), atrial fibrillation (5.7%), constipation (2.2%), gastritis (4.9%), diabetes mellitus (4.2%), myalgia (3.7%), headache (2.5%), insomnia (4.0%), vertigo (4.5%), bronchitis (6.6%), sinusitis (2.3%), eczema (4.5%), urinary tract infection (3.2%) |
| Familial hyper-cholesterolaemia | Simvastatin: 40mg once daily | HeFH: Class IC [2] | |||
| Prevention of cardiovascular events | 20-40mg once daily | Class IA [2] | |||
| Fibrates | Gemfibrozil | Hyperlipidaemias of types IIa, IIb, III, IV and V | Gemfibrozil: 0.9-1.2mg daily | Low HDL-C: Class IIbB; Transplant patients (with HTG, low HDL-C): Class IIbC [6] | Gastro-intestinal disturbances including dyspepsia (19.6%), nausea (4%), abdominal pain (9.8%), diarrhoea (7.2%), vomiting (1.2%); headache (1.2%), fatigue (3.8%), vertigo (1.5%), eczema, rash (1.7%), atrial fibrillation (0.7%), pancreatitis, appendicitis, disturbances in liver function including hepatitis and cholestatic jaundice, dizziness, paraesthesia, sexual dysfunction, thrombocytopenia, anaemia, leucopenia, eosinophilia, bone-marrow suppression, myalgia, myopathy, myasthenia, myositis accompanied by increase in creatine kinase, blurred vision, exfoliative dermatitis, alopecia, and photosensitivity |
| Ezetimibe | Primary and familial hyper-cholesterolaemia | 10mg once daily | Transplant patients (with high LDL-C): Class IIbC [6] | Gastro-intestinal disturbance including diarrhoea (4.1%) and abdominal pain (3.0%); headache, fatigue (2.4%); myalgia, arthralgia (3.0%), sinusitis (3.6%), pharyngitis (2.3%), viral infection (2.2%), coughing (2.3%), hypersensitivity reactions including rash, angioedema, and anaphylaxis, hepatitis,pancreatitis, cholelithiasis, cholecystitis, thrombocytopenia, raised creatine kinase, myopathy, and rhabdomyolysis | |
References
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, and ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012 Jul;33(14):1787-847. DOI:10.1093/eurheartj/ehs104 |
- Rydén L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, Cosentino F, Jönsson B, Laakso M, Malmberg K, Priori S, Ostergren J, Tuomilehto J, Thrainsdottir I, Vanhorebeek I, Stramba-Badiale M, Lindgren P, Qiao Q, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo J, Zamorano JL, Deckers JW, Bertrand M, Charbonnel B, Erdmann E, Ferrannini E, Flyvbjerg A, Gohlke H, Juanatey JR, Graham I, Monteiro PF, Parhofer K, Pyörälä K, Raz I, Schernthaner G, Volpe M, Wood D, Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC), and European Association for the Study of Diabetes (EASD). Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J. 2007 Jan;28(1):88-136. DOI:10.1093/eurheartj/ehl260 |
- Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvänne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F, European Association for Cardiovascular Prevention & Rehabilitation (EACPR), and ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012 Jul;33(13):1635-701. DOI:10.1093/eurheartj/ehs092 |
- Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van 't Hof A, Widimsky P, and Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012 Oct;33(20):2569-619. DOI:10.1093/eurheartj/ehs215 |
- Blomström-Lundqvist C, Scheinman MM, Aliot EM, Alpert JS, Calkins H, Camm AJ, Campbell WB, Haines DE, Kuck KH, Lerman BB, Miller DD, Shaeffer CW Jr, Stevenson WG, Tomaselli GF, Antman EM, Smith SC Jr, Alpert JS, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Hiratzka LF, Hunt SA, Jacobs AK, Russell RO Jr, Priori SG, Blanc JJ, Budaj A, Burgos EF, Cowie M, Deckers JW, Garcia MA, Klein WW, Lekakis J, Lindahl B, Mazzotta G, Morais JC, Oto A, Smiseth O, Trappe HJ, American College of Cardiology, American Heart Association Task Force on Practice Guidelines, and European Society of Cardiology Committee for Practice Guidelines. Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias). Circulation. 2003 Oct 14;108(15):1871-909. DOI:10.1161/01.CIR.0000091380.04100.84 |
- European Association for Cardiovascular Prevention & Rehabilitation, Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D, and ESC Committee for Practice Guidelines (CPG) 2008-2010 and 2010-2012 Committees. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011 Jul;32(14):1769-818. DOI:10.1093/eurheartj/ehr158 |